More Information
Submitted: March 03, 2025 | Approved: March 12, 2025 | Published: March 13, 2025
How to cite this article: Hoq M, Chang TMS. Effect of Polyhemoglobin and Synthetic Enzymes on Ischemic Cardiomyocytes. J Cardiol Cardiovasc Med. 2025; 10(2): 028-040. Available from:
https://dx.doi.org/10.29328/journal.jccm.1001207
DOI: 10.29328/journal.jccm.1001207
Copyright license: © 2025 Hoq M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Effect of Polyhemoglobin and Synthetic Enzymes on Ischemic Cardiomyocytes
Muntasirul Hoq and TMS Chang*
Artificial Cells & Organs Research Centre, Departments of Physiology, Medicine and Biomedical Engineering, Faculty of Medicine and Health Sciences, Montreal, QC, Canada
*Address for Correspondence: TMS Chang, Artificial Cells & Organs Research Centre, Departments of Physiology, Medicine and Biomedical Engineering, Faculty of Medicine and Health Sciences, Montreal, QC, Canada, Email: thomas.chang@mcgill.ca
We studied the effect of a novel solution consisting of polyhemoglobin together with three synthetic enzymes (neocatalase, neosuperoxide dismutase, and neocarbonic anhydrase) on ischemic human cardiomyocytes. Polyhemoglobin alone showed minimal protective effects. The addition of the three synthetic enzymes to polyhemoglobin provided antioxidant protection, reduced Reactive Oxygen Species (ROS) levels, and improved cellular recovery. ROS production and mitochondrial function assays confirmed significant reductions in oxidative damage with the combined treatment. Apoptosis and necrosis detection showed improved cell survival, while cardiac troponin assays indicated reduced myocardial injury. The novel solution of polyhemoglobin with all three synthetic enzymes provided the best protection compared to polyhemoglobin alone or polyhemoglobin combined with only two neoenzymes (neoCatalase and neoSuperoxide Dismutase). This study highlights the potential of polyhemoglobin with the three synthetic enzymes for the next generation blood substitutes and organ preservation as it offers a novel approach to improving the outcomes of ischemic cardiomyocytes.
The first artificial cells, reported by Dr. Thomas Chang in 1957, were not meant to replicate biological cells but used available basic knowledge to create a very simple system with possible medical applications [1]. Much of our knowledge about artificial cells is a direct result of research done by his group in the last 20 years. Many of the concepts that were presented in the original ideas are now used in the development of various modern medical fields, such as stem cell therapy, nanotechnology, and regenerative medicine [2]. In 2005, Dr. Chang published a review in Nature Review Drug Discovery where the editor prepared a table showing that Dr. Chang had made 20 major discoveries related to these areas up to that time [3]. Since then, other groups have also made significant progress and exciting new discoveries.
Progress in artificial cells has accelerated in the face of major advances in other areas. First, there has been the emergence of biomaterial, and then there has been the recognition of the importance of biotechnology. The progress in genomics and molecular biology has contributed to a leap in the field of artificial cells. In addition, there will be important developments in other areas such as artificial intelligence and nano-robots, which will contribute to the development of artificial cells [4].
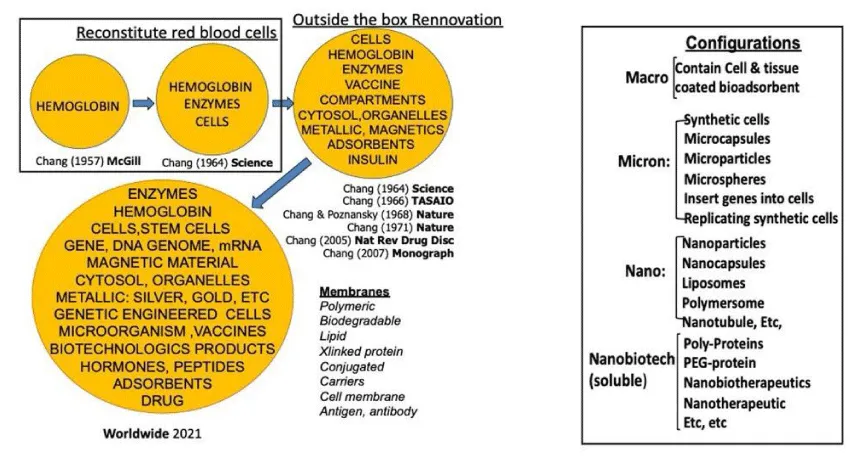
In 1972, Dr. Chang presented his monograph on artificial cells, where he stated that the concept of artificial cells was not a physical entity [5]. Instead, it was an idea that involved the creation of cellular structures that could be used to replace or supplement the functions of defective cells. The field of artificial cells has advanced so fast that this prediction needs to be updated. Due to the more recent advancements in the field of artificial cells, the contents and membranes of these structures have become more complex (Scheme 1). They can be categorized into three dimensions: macro, micro, and nano (Scheme 1). Researchers like to coin new names for their own modified configuration of artificial cells to differentiate them from others’ configurations. These have resulted in confusing new terminologies for someone who is new to this field.
Scheme 1: The top row (Left) shows some key ideas that led to the creation of different types of early artificial cells. The bottom row (Left) shows how far researchers have come with present-day artificial cells, which are made in a variety of forms and hold many different kinds of contents within their membranes. Artificial cells can be divided into four categories based on their size and structure (Right): macro-, micro-, nano- or soluble nanobiotechnologic. Each category comes with its own unique set of properties, applications -and terminology5 (With copyright permission from the author and publisher).
Dr. Chang first researched artificial cells with hemoglobin in 1957 [1,2]. Before 1989, little research focused on blood substitutes, with most studies exploring enzymes, adsorbent substances, and proteins [3-5]. In 1987, research on hemoglobin-based oxygen carriers gained interest due to concerns about HIV-contaminated donor blood [1,3,4]. Modified hemoglobin is useful in surgeries with extensive blood loss, like cancer and trauma surgeries [1]. It is especially beneficial in severe trauma injuries, such as those from car accidents and wars [1]. Unlike red blood cells, it does not need typing or cross-matching, making it ideal for emergencies. It can also be stored at room temperature for up to two years.
The most studied polyhemoglobin is Chang’s glutaraldehyde crosslinked soluble polyhemoglobin [6]. This crosslinking stabilizes hemoglobin and extensive research on this led to successful versions like human and bovine polyhemoglobin [7,8]. PolyHb has shown effectiveness in oxygen transport during clinical trials. Bovine polyhemoglobin from Biopure is approved in South Africa and Russia for elective surgeries to avoid HIV-contaminated blood [7]. Moore, et al. [8] tested human polyhemoglobin in ambulances for hemorrhagic shock. Patients receiving saline needed donor blood upon hospital arrival, while the polyhemoglobin group avoided transfusion for 14 hours. However, nonfatal myocardial infarction occurred in 0.6% of the control group and 3% of the polyhemoglobin group [8]. Natanson, et al. [9] concluded all HBOCs are cardiotoxic. To address this, Dr. Chang’s team developed PolyHb-CAT-SOD-CA by adding catalase, superoxide dismutase, and carbonic anhydrase [10]. This improved PolyHb reduced oxidative stress and improved CO₂ transport without cardiac side effects in hemorrhagic shock rats [10,11].
We are now studying the use of synthetic enzymes, like neo-catalase (neoCAT), neo-superoxide dismutase (neoSOD), and neo-carbonic anhydrase (neoCA), that mimic their natural counterparts. They are more stable and would cause fewer immune reactions as they are very small molecules [12,13]. In our earlier studies, combining these synthetic enzymes with PolyHb improved cell survival and lowered Reactive Oxygen Species (ROS) in ischemic hepatocytes [12,13]. These results show that synthetic enzymes can boost the protective effects of PolyHb. They also provide a more stable and adaptable way to manage ischemic injuries.
Cardiomyocytes, the heart’s main contractile cells, are very sensitive to ischemic injury. These cells have high energy needs, which makes them prone to damage. In ischemic conditions, a lack of oxygen and nutrients leads to oxidative stress, cell death, and functional loss. During reperfusion, the sudden return of oxygen increases ROS production. This causes even more damage to the cells. Moreover, the ischemic heart was linked with high intracellular pCO2 [14]. Furthermore, cardiomyocytes are especially vulnerable because they depend on constant mitochondrial activity to keep contracting and maintain electrical signaling. Our earlier studies showed that PolyHb solutions with synthetic enzymes can reduce ischemic damage in hepatocyte cultures [12,13]. Now, we wanted to see if the combination of polyHb and the three neoenzymes could protect the cardiomyocytes. These cells are vital for heart function and are more vulnerable to ischemic injury due to elevated tissue pCO₂ and oxidative stress. Preliminary results showed that combining PolyHb with synthetic enzymes, including neoSOD, neoCAT, and neoCA, improved cardiomyocyte survival. It also lowered oxidative stress markers after ischemic shock [13]. These findings support the need for a detailed study on how this solution protects cardiomyocytes.
In the present study, we focus on testing PolyHb combined with neoSOD, neoCAT, and neoCA to protect cardiomyocytes from ischemic injury. This is a more direct test on the effect of polyHb + 3 neoenzymes on the heart cells. In this form, we are not dealing with the microcirculation and other complex systemic factors. We will measure oxidative stress, apoptosis and necrosis, mitochondrial function, cell viability, and function. This research aims to explain how this combination works to protect cardiomyocytes. The goal is to improve the preservation of cardiomyocytes under ischemic conditions. Success in this area could lead to better next-generation blood substitutes which could also be used as pre-transplantation preservation fluid.
Reagents and cell line
All chemical reagents were purchased from Sigma Aldrich Canada including MnTmPyP (CAT# 475872) and EUK-134 (CAT# SML0743). Human cardiomyocytes (C-12810) and their media (C-22070) were also purchased from Sigma Aldrich.
Synthesis of neo-Carbonic Anhydrase (neoCA)
NeoCA was synthesized using a published protocol [15]. Zinc chloride (0.51 g) and L-histidine (1.72 g) were mixed with 4.8 mL of glycerol in a beaker. The mixture was stirred at 70 °C until a light yellow liquid formed. The mixture was then dried in a vacuum oven at 60 °C for 2 hours. This produced ZnHisGly, also called neoCA.
polyHb preparation
PolyHb was prepared using a published protocol [10]. A beaker was flushed with nitrogen gas and kept on ice to prevent metHb formation. Ultrapure hemoglobin (4 mL, 10 g/dL) was added to the beaker. Sodium chloride (102 μL, 4 M) and potassium phosphate buffer (102 μL, 3 M) were added. Lysine (229 μL, 2 M) was then added and the mixture was shaken at 160 rpm at 4 °C for 5 minutes. Glutaraldehyde (162.4 μL, 0.5 M) was added in four equal parts over 15 minutes while shaking. The mixture was shaken overnight at 160 rpm at 4 °C. Next, lysine (656 μL, 2 M) was added in two equal parts over 10 minutes to stop crosslinking. The mixture was shaken at 160 rpm for another hour at 4 °C. The sample was then centrifuged at 8000 rpm for 1 hour at 4 °C to remove precipitates. The supernatant was purified using a Vivaspin 20 centrifugal filter (CAT# GE28-9323-60, 100 kDa cutoff) at 2500 g for 55 minutes at 4 °C. The retentate-containing polyHb with less than 2% tetrameric Hb, was collected and stored at -20 °C for further use.
Preparation of a solution containing polyHb + 3 neoenzymes
The solution was prepared by mixing 1 g of polyHb with approximately 18 mg each of neoSOD, neoCAT, and neoCA.
Human cardiomyocyte culture
PromoCell Myocyte Growth Medium (C-22070, Sigma Aldrich Canada) supplemented with 5% FBS and 1% penicillin/streptomycin was warmed to 37 °C in a water bath. Cryopreserved cardiomyocytes were thawed in a 37 °C water bath for less than 2 minutes. The vial was then wiped with 70% alcohol inside a hood. Its content was then transferred to the warm medium. The cells were then seeded at a density of 10,000 cells per cm2. The plates were then incubated at 37 °C.
Experimental protocol for warm ischemia model
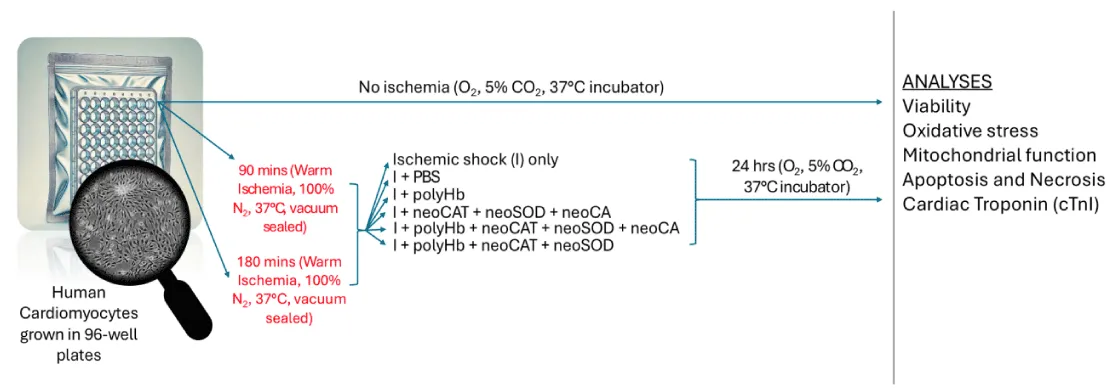
The plates were at first individually vacuum-sealed using a vacuum sealer. Each vacuum-sealed bag contained a silica gel packet as well. The plates were then placed in a glove bag with a continuous nitrogen gas flow. A vacuum outlet was used to remove residual air, creating an in vitro ischemic environment. Two experimental sets were prepared: one subjected to 90 minutes of ischemic shock and the other to 180 minutes. After ischemic shock, the cells were treated with the following combinations (Scheme 2):
Scheme 2: Human cardiomyocytes were cultured in 96-well plates. Cells were subjected to warm ischemia for 90 or 180 minutes (100% N₂, 37 °C, vacuum-sealed). Ischemic cells were given different treatments like PBS, polyHb, 3 neoenzymes, polyHb + 3 neoenzymes, or polyHb + neoSOD + neoCAT. After treatment, cells were incubated for 24 hours (5% CO₂, 37°C). Analyses included viability, oxidative stress, mitochondrial function, apoptosis, necrosis, and cardiac troponin I (cTnI). Non-ischemic controls were maintained under normal conditions.
- Ischemic shock (I) control:Treated with only fresh media after ischemic shock.
- I + PBS: Treated with 4 µL PBS (RL) and fresh media.
- I + polyHb: Treated with 0.626 mmol/L PolyHb (1 g/dL) and fresh media.
- I + 3 neoenzymes: Treated with 55.6 mmol/L of all 3 neoenzymes (neoSOD: 2960 U/mL, neoCAT: 304411 U/mL, neoCA: 360 W-A U/mL) and fresh media.
- I + polyHb + 3 neoenzymes: Treated with 0.626 mmol/L PolyHb (1 g/dL) and 55.6 mmol/L of all 3 neoenzymes (neoSOD: 2960 U/mL, neoCAT: 304411 U/mL, neoCA: 360 W-A U/mL) in fresh media.
- I + polyHb + 2 neoenzymes: Treated with 0.626 mmol/L PolyHb (1 g/dL) and 55.6 mmol/L of 2 neoenzymes (neoSOD: 2960 U/mL, neoCAT: 304411 U/mL) in fresh media.
The plates were then returned to the incubator and kept for an additional 24 hours.
MetHb reduction
The reduction of metHb was done following the protocol described previously. Briefly, a 3% (w/v) bovine PolyHb was prepared in a 0.05M phosphate phosphate buffer solution (pH 7.4) at first. After that, a solution of 1M potassium ferricyanide was added to the above PolyHb solution to oxidize the iron in PolyHb from a ferrous (Fe2+) state to a ferric state (Fe3+). This mixture was then dialyzed through a 6-8 kDa MWCO membrane in a 0.05M PBS solution (pH 7.4) to get rid of the excess potassium ferricyanide from the mixture. Then, a solution of 10% (w/v) Vitamin C was prepared and a certain amount of it was added into the dialyzed metHb solution to get Vitamin C in the solution with a final concentration of 0.05% (w/v). Vitamin C addition would then trigger the reduction reaction and the metHb content was tested periodically over a time of 24 hrs using a UV spectrophotometer at 630nm till the metHb level became stable.
Dosage response curve
The MTT assay was conducted to generate a dosage response curve. This was done to assess the cell viability at varying concentrations of the test compound i.e., polyHb + 3 neoenzymes. Initially, MTT reagent was prepared by dissolving 50 mg of MTT in 10 mL of PBS (pH 7.4) and sterile-filtered for use. After 90 min of ischemic shock followed by different treatments, the cells were incubated for 24 hrs. Then, 50 µL of the prepared MTT solution was added to each well, and the plates were incubated at 37 °C for 4 hours to allow for the formation of MTT formazan crystals within the cells. After incubation, the media was carefully removed, and 500 µL of isopropanol with 0.04N HCl was added to each well to dissolve the formazan crystals. The resulting solutions were transferred to cuvettes, and absorbance was measured at 570 nm using a spectrophotometer. This procedure helped create a dosage response curve by measuring absorbance values that show cell viability at different concentrations of the test compound.
Reactive Oxygen Species (ROS) production assay
Oxidative stress is measured by detecting Reactive Oxygen Species (ROS) through live-cell imaging. This method provides real-time data on ROS levels. The cells were stained with CellROX Green, which binds to ROS, and Hoechst 33342, which stains DNA. Imaging was done under 20X magnification using a fluorescent microscope at 485/520 nm. The overlap of staining in nuclei was analyzed to determine total nuclei and CellROX-positive nuclei. These percentages were then used to create a graphical representation.
Mitochondrial function assay
The cardiomyocytes in 96-well plates were washed with 100 µL of PBS per well to remove residual media. Then, 100 µL of 1 µM JC-1 working solution was added to each well, ensuring the cells were fully covered. The plate was then incubated at 37 °C for 10 minutes in the dark to allow the JC-1 dye to stain the mitochondria. After incubation, the JC-1 solution was removed, and the cells were washed twice with 100 µL of PBS or 1X Dilution Buffer to eliminate excess dye. Following the washes, 200 µL of 1X Dilution Buffer was added to each well, and fluorescence was measured. Imaging was done under 20X magnification using a fluorescent microscope to detect red fluorescence (JC-1 aggregates) at excitation 535 nm and emission 590 nm and green fluorescence (JC-1 monomers) at excitation 475 nm and emission 530 nm. Red and green fluorescent cells were counted, and the red/green fluorescence ratio was calculated. A higher ratio was interpreted as indicating healthier mitochondria, while a lower ratio suggested mitochondrial dysfunction.
Apoptosis and necrosis detection assay
For harvesting cells, media was removed followed by washing with PBS and detaching with trypsin-EDTA. Trypsin was neutralized with complete media. The detached cells were centrifuged at 400 x g for 5 minutes. The cell pellet was resuspended in a 1X binding buffer. Then, 5 μL of both Annexin V-FITC and PI were added. Cells were then incubated for 10 minutes in the dark. It was then washed with binding buffer and centrifuged again. The cells were resuspended and visualized under a fluorescence microscope using FITC (excitation at 488 nm, emission at 530 nm) and PI (excitation at 535 nm, emission at 617 nm) filters. Live cells showed no fluorescence. Early apoptotic cells showed green fluorescence. Late apoptotic or necrotic cells showed both green and red fluorescence. Necrotic cells showed red fluorescence only.
Cardiac Troponin I (cTnI) assay
The culture media were collected from each well after 24 hours of incubation following ischemic shock and treatments. The media were centrifuged at 500 x g for 10 minutes to remove debris. The supernatant was transferred to fresh tubes for analysis. A cTnI ELISA kit was used following the manufacturer’s instructions. The detection antibody was added to the wells and incubated at 37 °C for the specified time. The wells were washed with 1X wash buffer after incubation. The substrate solution was added to the wells and incubated at room temperature in the dark until color developed. The reaction was stopped using the stop solution provided in the kit. The absorbance was measured at 450 nm using a microplate reader. The cTnI concentrations were calculated using a standard curve generated from known concentrations.
Data analysis
The data were presented as mean ± standard deviation (SD). Differences between the control, RL, polyHb, and polyHb + neoenzymes groups were analyzed using one-way ANOVA. A significance level of 95% was used for all tests. The Newman-Keuls test was performed to identify specific groups with statistically significant differences.
Vitamin C addition to polyHb resulted in a significant reduction of methemoglobin (metHb) content
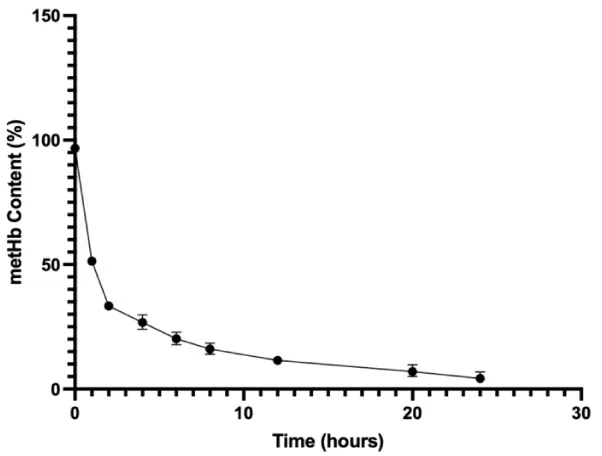
MetHb content after Vitamin C addition was calculated in comparison to the metHb content prior to Vitamin C addition (about 100%) as can be seen in Figure 1. The metHb content decreased from 96.71 ± 0.67% to 5.12 ± 1.67% over the period of 24hrs. This indicates that the metHb could be reduced in the presence of Vitamin C, both effectively and quickly.
Figure 1: Bovine metHb reduction using Vitamin C over a period of 24 hrs.
Dosage response curve construction using MTT assay
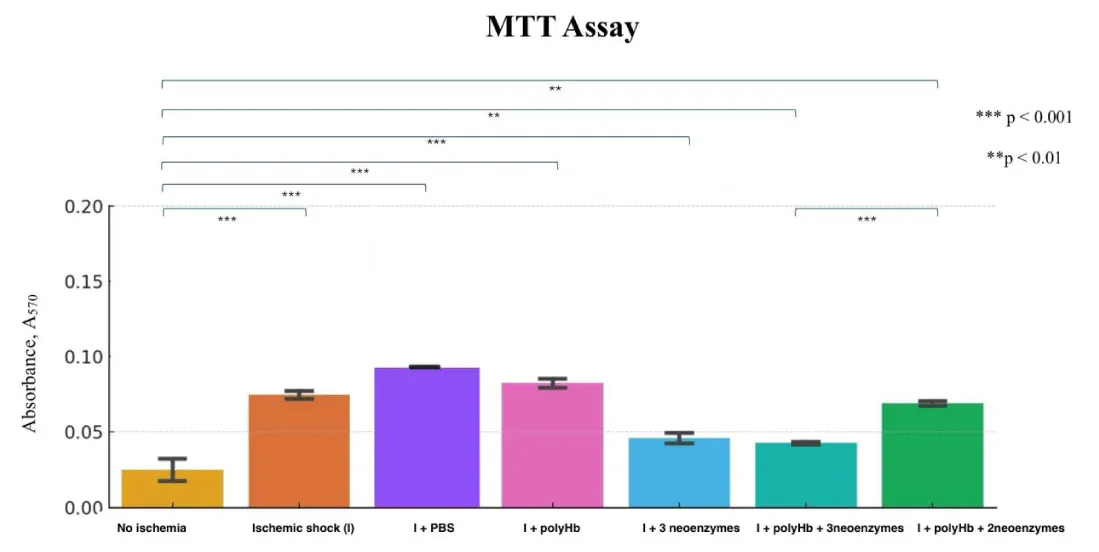
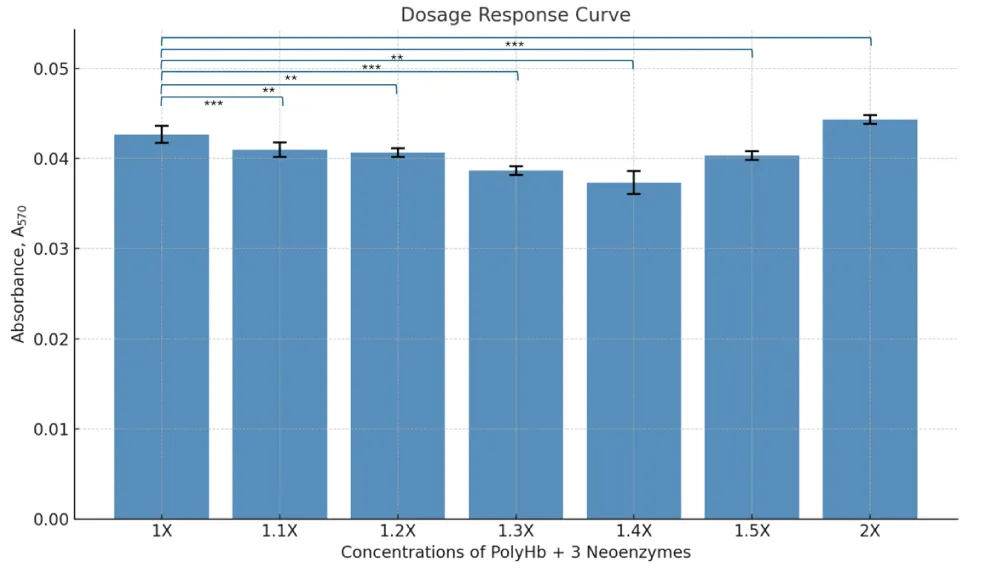
The MTT assay results showed that polyHb + 3 neoenzymes preserved cell viability almost as well as the no ischemia group, which had no ischemic shock (Figure 2). The absorbance values for the ischemic shock (I) group (ischemic shock and no other treatment) were significantly higher than the no ischemia group, indicating more cell death. The group I + RL showed the highest level of cell death. The dosage response curve revealed that the best results were at a concentration of 1.4x polyHb + 3 neoenzymes (Figure 3). At this dose, cell viability was highest, showing strong protection against ischemic shock. Statistical analysis confirmed significant differences between the negative control and all other treatments.
Figure 2: MTT assay showing cell viability (A₅₇₀) across treatments: No Ischemia control, Ischemic shock (I) control, I+ PBS, I + polyHb, I + 3 neoenzymes, I + polyHb + 3 neoenzymes, and I + polyHb + 2 neoenzymes (neoSOD + neoCAT). ***p < 0.001, **p < 0.01.
Figure 3: Dose-response curve showing cell viability (A₅₇₀) at varying concentrations (1X–2X) of polyHb combined with 3 neoenzymes.
Reactive Oxygen Species (ROS) production was effectively mitigated by polyHb + 3 neoenzymes in both 90min and 180min Ischemic groups
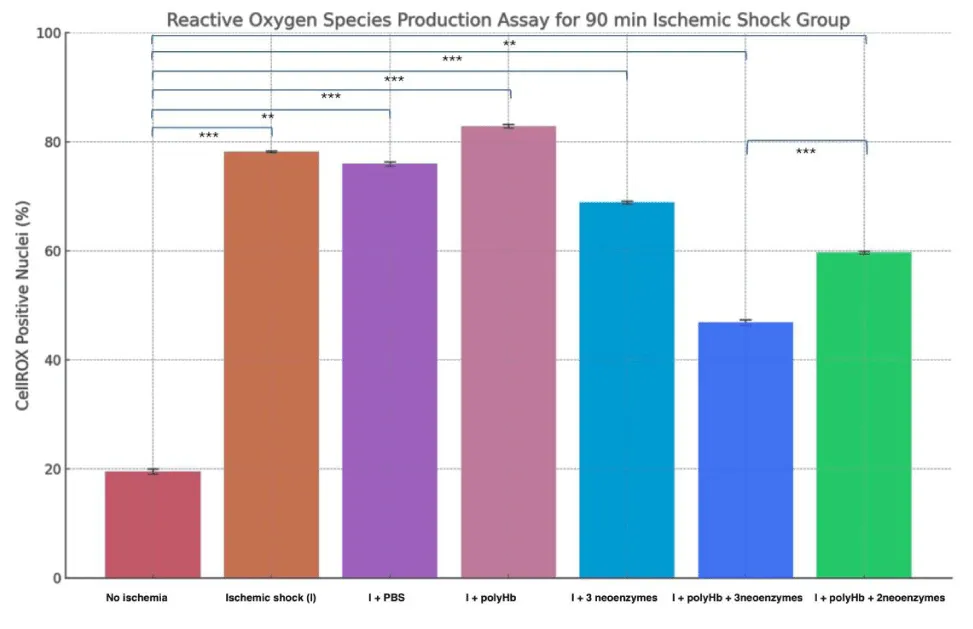
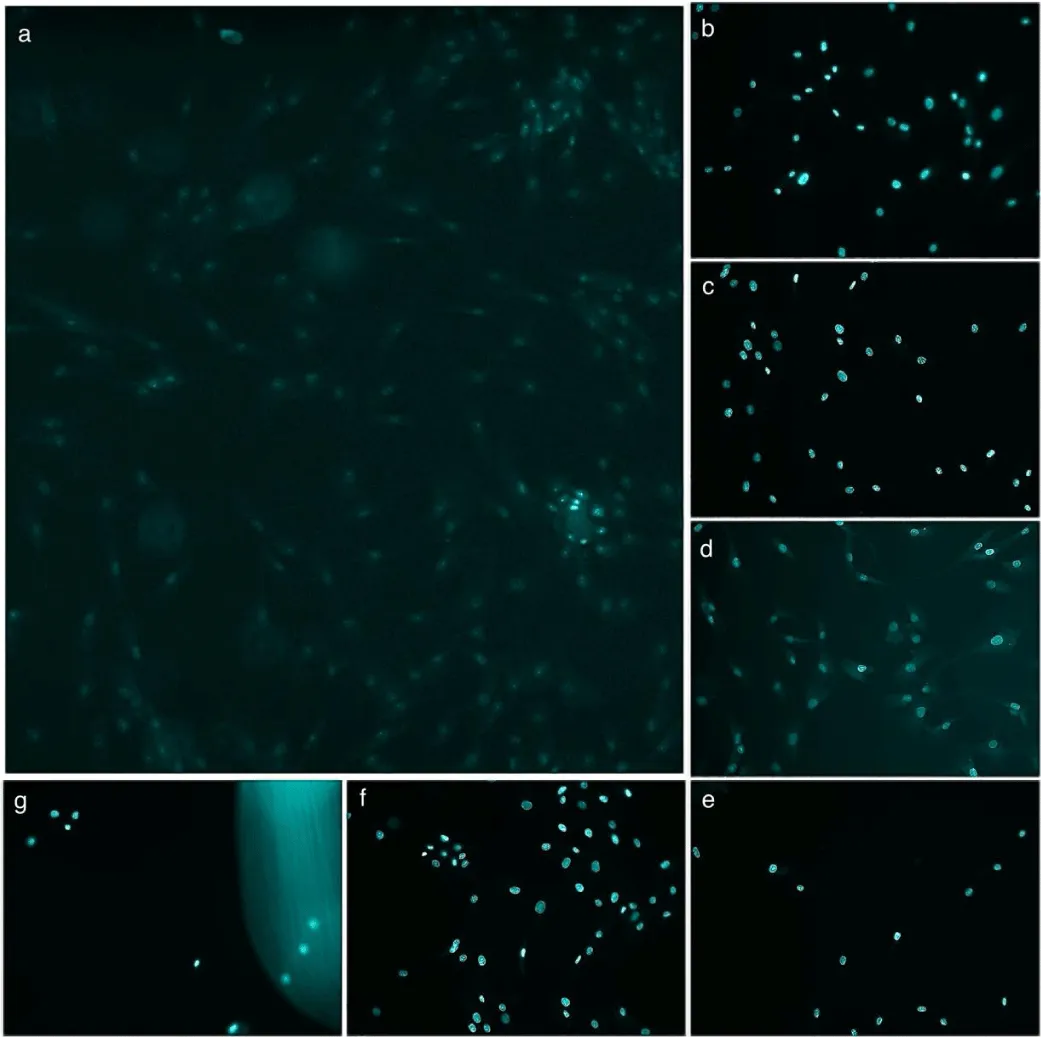
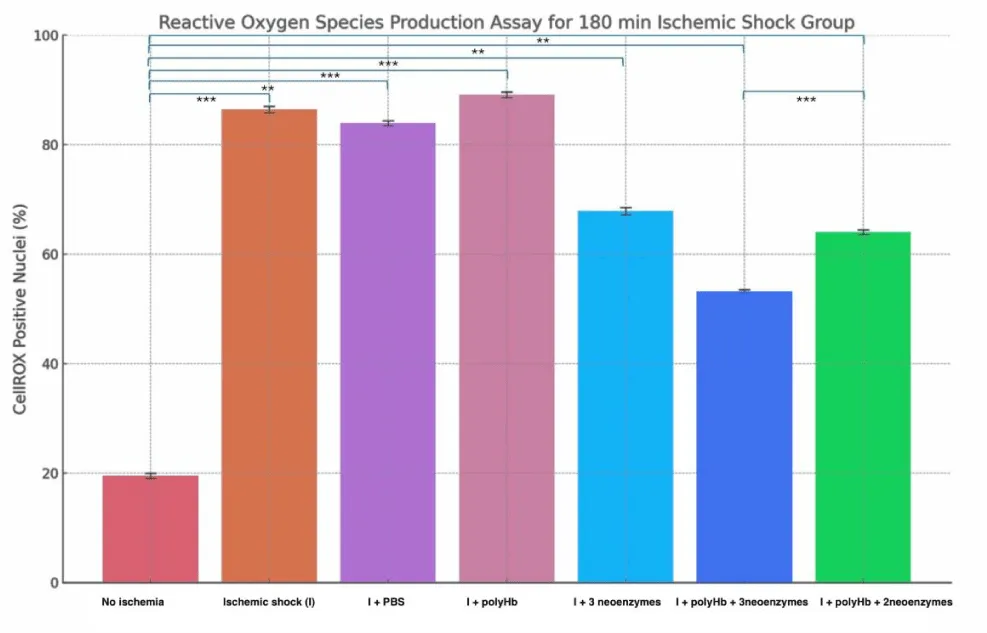
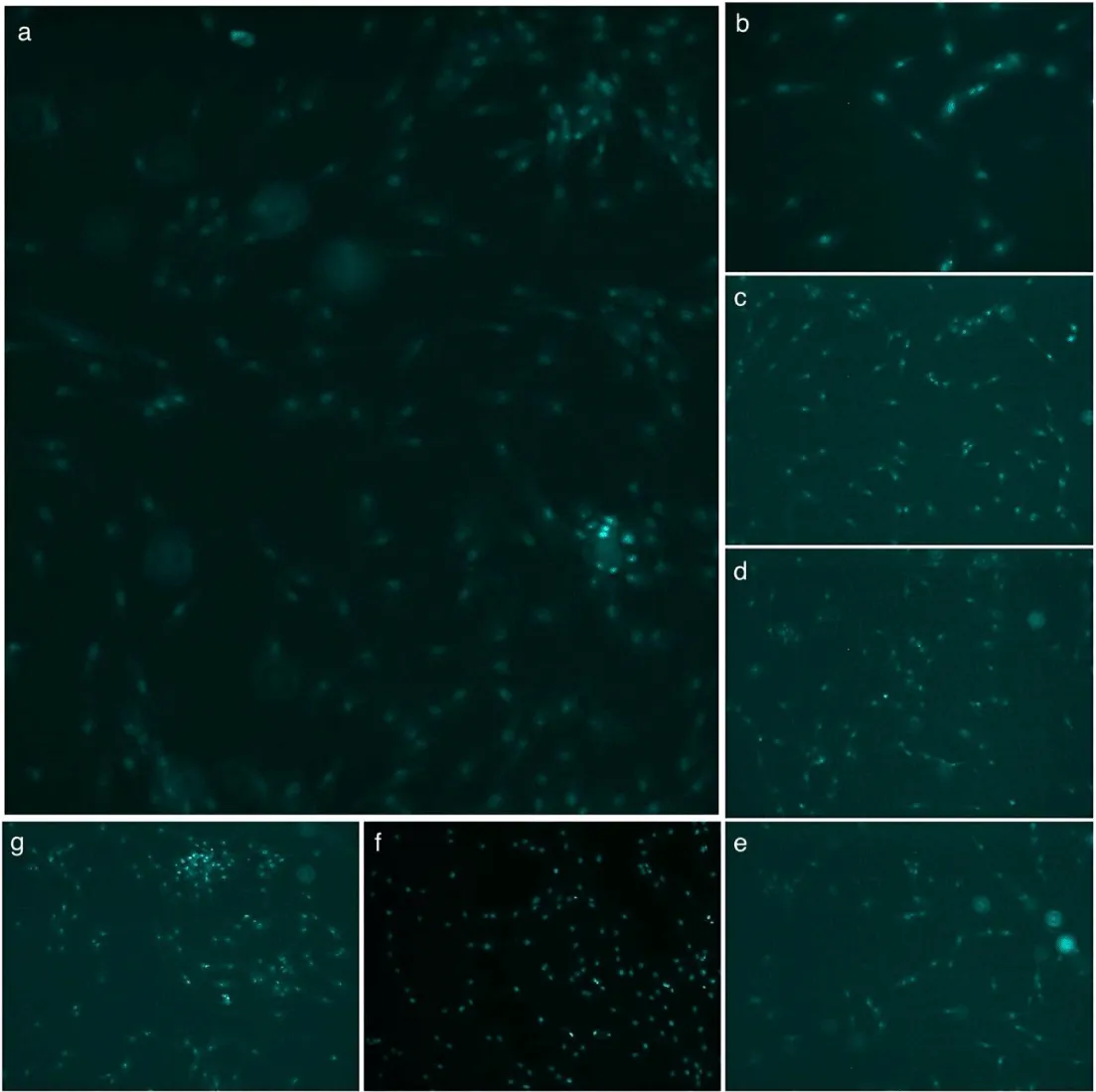
Figure 4 shows the percentage of CellROX-positive nuclei in cardiomyocytes under different treatments after 90 minutes of Ischemia (Figure 5 shows the DAPI and CellROX merged images for the 90-minute group). The “No Ischemia” group had the lowest ROS levels (~20%), while the “Ischemic shock (I)” group showed the highest (~78%). Treatment with PBS or polyHb alone did not significantly reduce ROS levels compared to I. However, “I + 3 neoenzymes” lowered ROS to ~69%, and “I + polyHb + 3 neoenzymes” achieved the most significant reduction (~47%). The “I + polyHb + 2 neoenzymes (neoSOD + neoCAT)” group showed moderate ROS reduction (~59%). Figure 6 shows ROS levels in cardiomyocytes after 180 minutes of ischemic shock and Figure 7 shows the DAPI and CellROX merged images. The “No Ischemia” group had the lowest ROS levels (~20%), while the “I” group showed the highest (~87%). The “I + PBS” group had slightly lower ROS levels (~84%) compared to I, but the difference was minimal. The “I + polyHb” group showed the highest ROS among treated groups (~89%). The “I + 3 neoenzymes” group reduced ROS significantly (~68%), while the “I + polyHb + 3 neoenzymes” group achieved the lowest ROS (~53%) among treated groups. The “I + polyHb + 2 neoenzymes (neoSOD + neoCAT)” group showed moderate ROS levels (~64%).
Figure 4: Reactive Oxygen Species (ROS) production assay for the 90-minute ischemic shock group. The results highlight the impact of different treatments on ROS levels in cardiomyocytes after ischemic shock. Statistical significance between groups is indicated as ***p < 0.001 and **p < 0.01.
Figure 5: Merged CellROX green and DAPI images showing CellROX-positive nuclei in cardiomyocytes after 90 minutes of ischemic shock. (a) No ischemia, (b) Ischemic shock (I), (c) I + PBS, (d) I + polyHb, (e) I + 3 neoenzymes, (f) I + polyHb + 3 neoenzymes, and (g) I + polyHb + 2 neoenzymes (neoSOD + neoCAT). The images highlight the effect of different treatments on reactive oxygen species (ROS) production, as indicated by CellROX-positive nuclei (Scale bar: 50um).
Figure 6: Reactive Oxygen Species (ROS) production assay for the 180-minute ischemic shock group. The results highlight the impact of different treatments on ROS levels in cardiomyocytes after ischemic shock. Statistical significance between groups is indicated as ***p < 0.001 and **p < 0.01.
Figure 7: Merged CellROX green and DAPI images showing CellROX-positive nuclei in cardiomyocytes after 180 minutes of ischemic shock. (a) No ischemia, (b) Ischemic shock (I), (c) I + PBS, (d) I + polyHb, (e) I + 3 neoenzymes, (f) I + polyHb + 3 neoenzymes, and (g) I + polyHb + 2 neoenzymes (neoSOD + neoCAT). The images highlight the effect of different treatments on reactive oxygen species (ROS) production, as indicated by CellROX-positive nuclei (Scale bar: 50um).
Combining polyHb with all three neoenzymes restores mitochondrial function most effectively in both 90min and 180min Ischemic groups
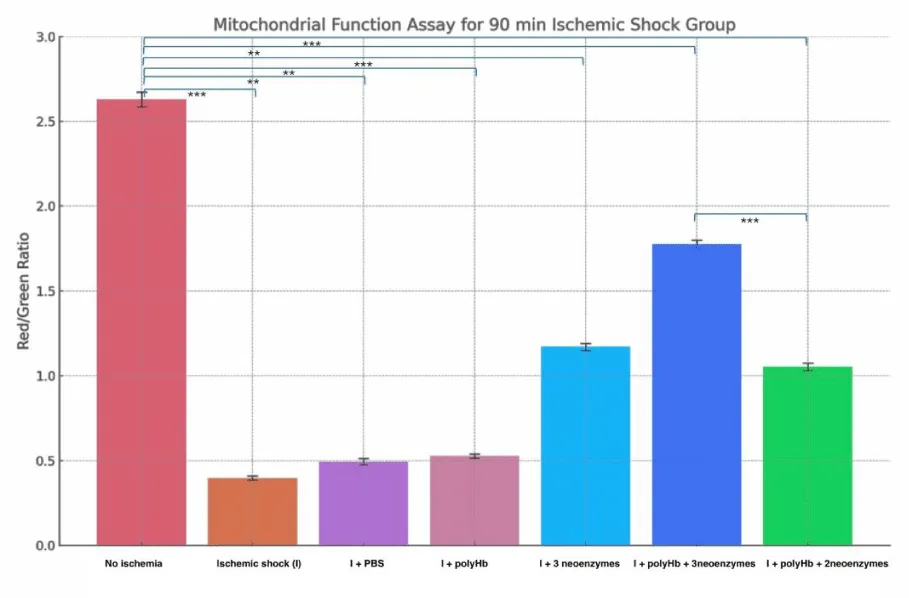
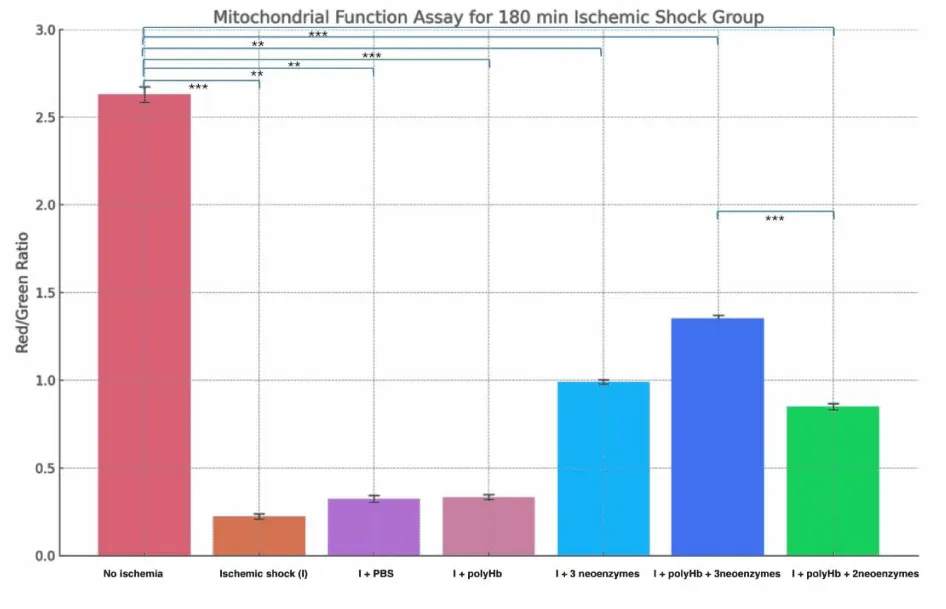
Figure 8 shows the Red/Green ratio of mitochondrial function in cardiomyocytes after 90 minutes of ischemic shock. The «No Ischemia» group had the highest ratio (~2.6), showing healthy mitochondrial function. The «Ischemic shock (I)» group had the lowest ratio (~0.4), indicating severe mitochondrial damage. The «I + PBS» and «I + polyHb» groups showed slight improvements but remained low (~0.5). The «I + 3 neoenzymes» group had a moderate recovery (~1.2). The «I + polyHb + 3 neoenzymes» group showed the best improvement (~1.8), while the «I + polyHb + 2 neoenzymes (neoSOD + neoCAT)» group showed moderate recovery (~1.1). Figure 9 shows the Red/Green fluorescence ratio for mitochondrial function in cardiomyocytes after 180 minutes of ischemic shock. The «No Ischemia» group had the highest ratio (~2.6), showing healthy mitochondria. The «I» group had the lowest ratio (~0.2), indicating severe mitochondrial damage. The «I + PBS» and «I + polyHb» groups showed slight improvements, with ratios of ~0.3. The «I + 3 neoenzymes» group moderately improved the ratio (~1.0). The «I + polyHb + 3 neoenzymes» group showed the best recovery, with a ratio of ~1.3. The «I + polyHb + 2 neoenzymes (neoSOD + neoCAT)» group showed moderate recovery, with a ratio of ~0.8.
Figure 8: Mitochondrial Function Assay for the 90-minute ischemic shock group. Bars represent the Red/Green fluorescence ratio under different treatment conditions: No Ischemia, Ischemic shock group (I), I + PBS, I + polyHb, I + 3 neoenzymes, I + polyHb + 3 neoenzymes, and I + polyHb + 2 neoenzymes (neoSOD + neoCAT). Statistical significance between groups is indicated as ***p < 0.001 and **p < 0.01.
Figure 9: Mitochondrial Function Assay for the 180-minute ischemic shock group. Bars represent the Red/Green fluorescence ratio under different treatment conditions: No Ischemia, Ischemic shock group (I), I + PBS, I + polyHb, I + 3 neoenzymes, I + polyHb + 3 neoenzymes, and I + polyHb + 2 neoenzymes (neoSOD + neoCAT). Statistical significance between groups is indicated as ***p < 0.001 and **p < 0.01.
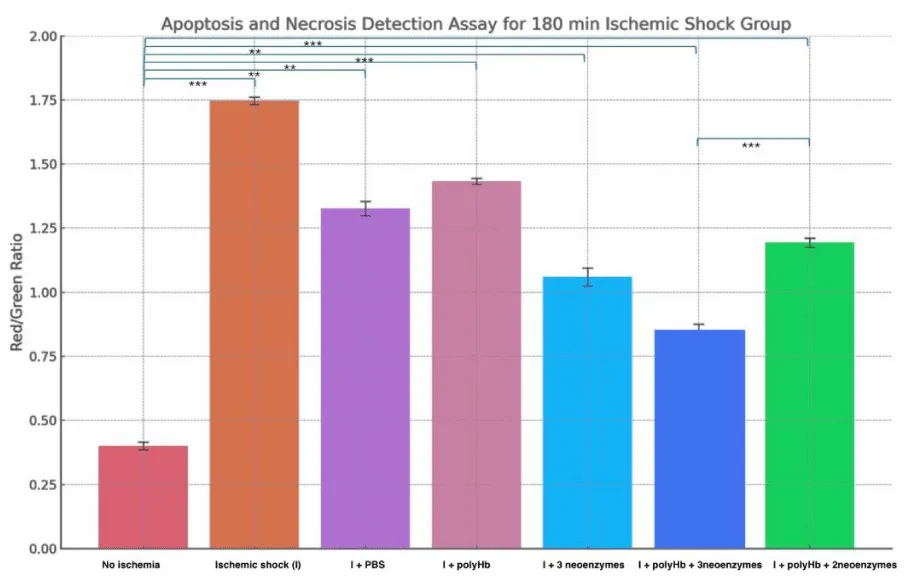
PolyHb + 3 neoenzymes provided effective protection against cell death in both 90min and 180min Ischemic groups
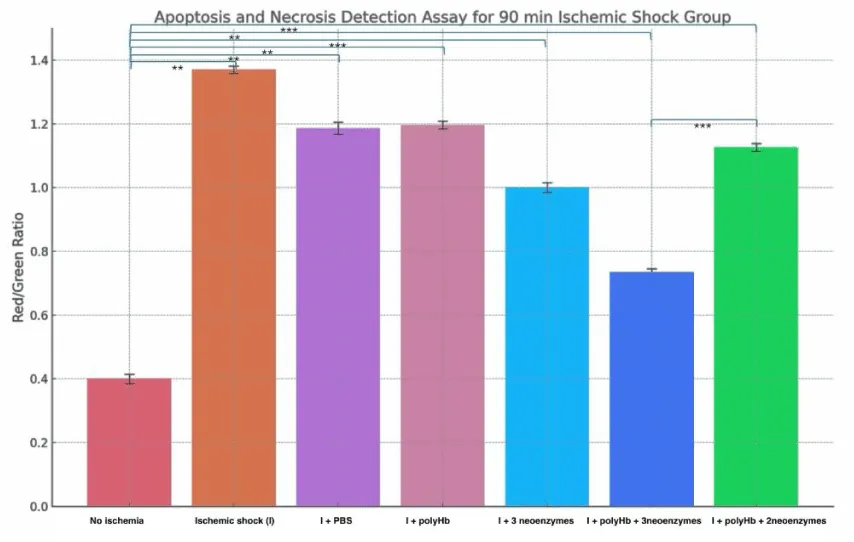
Figure 10 shows the effect of treatments on apoptosis and necrosis after 90 minutes of ischemic shock. The “No Ischemia” group had the lowest Red/Green ratio at around 0.4, indicating minimal cell death. The “Ischemic shock (I)” group had the highest ratio at about 1.7, reflecting severe necrosis. The “I + PBS” and “I + polyHb” groups showed moderate ratios near 1.3 and 1.4. The “I + 3 neoenzymes” group improved the ratio to around 1.0. The “I + polyHb + 3 neoenzymes” group had the lowest ratio among treatments at about 0.8, showing the best recovery. Figure 11 displays a similar analysis after 180 minutes of ischemic shock. The “No Ischemia” group again had the lowest ratio at around 0.4. The “I” group showed an even higher ratio of about 1.8, indicating more severe necrosis. The “I + PBS” and “I + polyHb” groups showed ratios near 1.3 and 1.4, similar to the 90-minute results. The “I + 3 neoenzymes” group had a ratio of about 1.1, while the “I + polyHb + 3 neoenzymes” group showed the lowest ratio at 0.9, indicating the best protection. These results highlight that the combination of polyHb with all three neoenzymes provided consistent and effective protection against cell death in both ischemic shock durations.
Figure 10: Apoptosis and Necrosis Detection Assay for the 90-minute ischemic shock group. Bars represent the Red/Green fluorescence ratio, which indicates the proportion of necrotic cells to early apoptotic cells under different treatment conditions: No Ischemia, Ischemic shock (I), I + PBS, I + polyHb, I + 3 neoenzymes, I + polyHb + 3 neoenzymes, and I + polyHb + 2 neoenzymes (neoSOD + neoCAT). A higher Red/Green ratio reflects increased necrosis, while a lower ratio indicates reduced necrosis and improved cellular health. Statistical significance between groups is indicated as ***p < 0.001 and **p < 0.01.
Figure 11: Apoptosis and Necrosis Detection Assay for the 180-minute ischemic shock group. Bars represent the Red/Green fluorescence ratio, which indicates the proportion of necrotic cells to early apoptotic cells under different treatment conditions: No Ischemia, Ischemic shock (I), I + PBS, I + polyHb, I + 3 neoenzymes, I + polyHb + 3 neoenzymes, and I + polyHb + 2 neoenzymes (neoSOD + neoCAT). Statistical significance between groups is indicated as ***p < 0.001 and **p < 0.01.
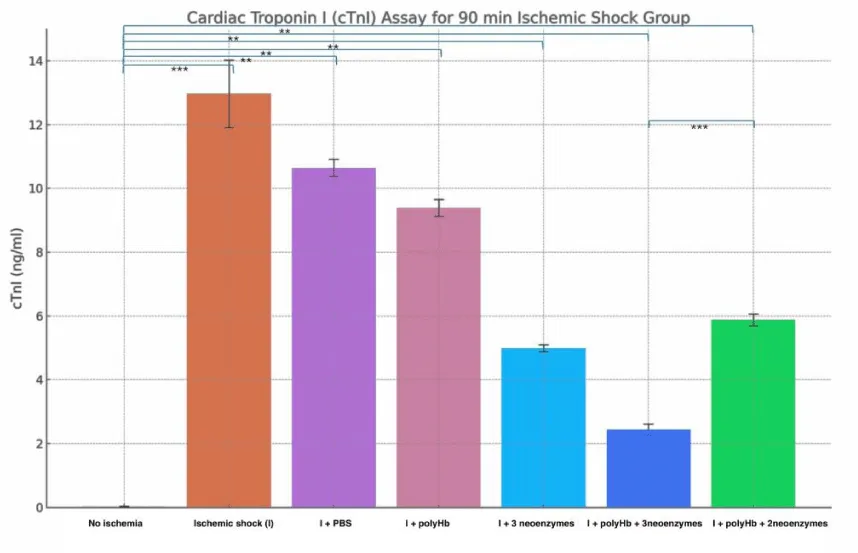
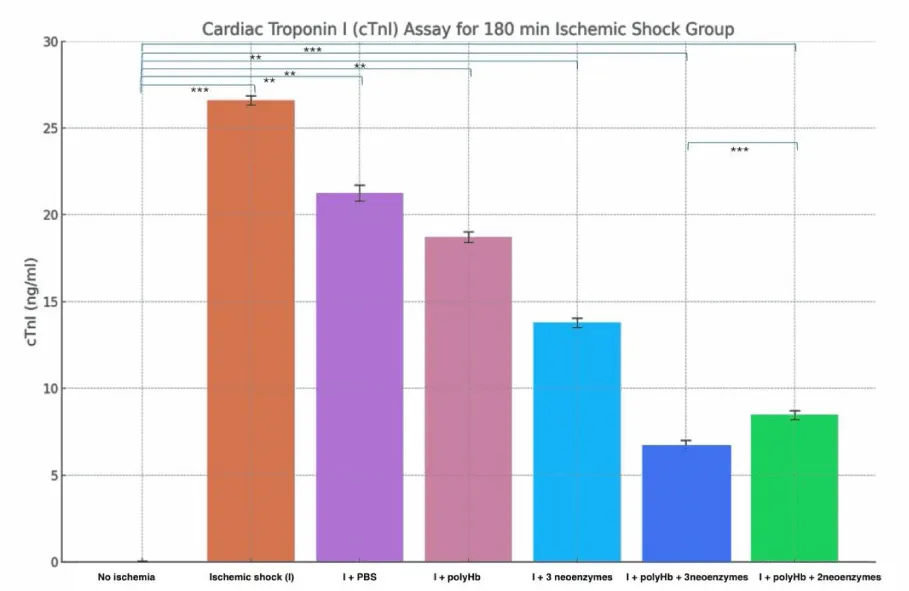
PolyHb + 3 neoenzymes effectively reduced cardiac injury in both 90min and 180min Ischemic groups
The results from the two figures (Figures 12,13) show the levels of cardiac troponin I (cTnI) as an indicator of cardiac injury after ischemic shock. In the 90-minute ischemic shock group, the «No Ischemia» condition showed the lowest cTnI levels at around 0.02 ng/ml. The «Ischemic shock (I)» group showed the highest levels at about 13 ng/ml reflecting severe cardiac damage. The «I + PBS» and «I + polyHb» groups showed moderate reductions in cTnI levels to around 10 and 9 ng/ml. The «I + 3 neoenzymes» group showed a further reduction to about 5 ng/ml. The «I + polyHb + 3 neoenzymes» group had the lowest levels at about 2.4 ng/ml showing the best protection. The 180-minute ischemic shock group showed similar trends but with higher cTnI levels due to longer ischemic exposure. The «No Ischemia» group had the lowest levels at around 0.02 ng/ml. The «I» group showed the highest levels at about 27 ng/ml. The «I + PBS» and «I + polyHb» groups reduced cTnI to around 21 and 19 ng/ml. The «I + 3 neoenzymes» group reduced levels to about 14 ng/ml. The «I + polyHb + 3 neoenzymes» group had the lowest levels at about 7 ng/ml showing the most significant protection. These results indicate that combining polyHb with all three neoenzymes effectively reduced cardiac injury in both ischemic shock groups.
Figure 12: Cardiac Troponin I (cTnI) Assay for the 90-minute ischemic shock group. Bars represent the mean cTnI levels (ng/ml) for different treatments: No Ischemia, Ischemic shock (I), I + PBS, I + polyHb, I + 3 neoenzymes, I + polyHb + 3 neoenzymes, and I + polyHb + 2 neoenzymes (neoSOD + neoCAT). Higher cTnI levels reflect greater cardiac damage, while lower levels indicate improved protection and recovery. Statistical significance between groups is indicated as ***p < 0.001 and **p < 0.01.
Figure 13: Cardiac Troponin I (cTnI) Assay for the 180-minute ischemic shock group. Bars represent the mean cTnI levels (ng/ml) for different treatments: No Ischemia, Ischemic shock (I), I + PBS, I + polyHb, I + 3 neoenzymes, I + polyHb + 3 neoenzymes, and I + polyHb + 2 neoenzymes (neoSOD + neoCAT). Higher cTnI levels reflect greater cardiac damage, while lower levels indicate improved protection and recovery. Statistical significance between groups is indicated as ***p < 0.001 and **p < 0.01.
PolyHb combined with synthetic enzymes (neoCAT, neoSOD, and neoCA), aims to mimic and enhance the natural functions of red blood cells. Ischemic hearts often have high levels of intracellular pCO2 [14]. This increase is a result of disrupted cellular respiration. Clinical trials showed that polyHb alone was linked to a risk of myocardial infarction [8]. Dr. Chang and his group crosslinked polyHb with catalase, superoxide dismutase, and carbonic anhydrase enzymes. This combination, polyHb-CAT-SOD-CA, reduced ischemia-related damage [10,11]. It helped manage pCO₂ levels and reduced oxidative stress in animal models. These results suggest that polyHb-CAT-SOD-CA could be a better solution for ischemic injury.
This study was performed to investigate the potential of combining polyHb with three synthetic enzymes (neoSOD, neoCAT, and neoCA) to mitigate ischemic injury in human cardiomyocytes. In our previous detailed study on hepatocytes, we found that the combination of polyHb and the three neoenzymes provided maximum cellular preservation after prolonged ischemic shocks [12,13]. Cardiomyocytes were chosen as the model for this study because ischemic damage to the heart is a major contributor to cardiac infarction. The aim was to determine whether combining all three enzymes with polyHb could enhance cellular recovery and whether neoCA plays a critical role in improving outcomes for cardiomyocytes. This study evaluated the impact of these treatments on oxidative stress, mitochondrial function, cell death, and cardiac damage. This would then provide us with a comprehensive understanding of the protective effects of the different treatments we used in this study.
To begin with, we analyzed methemoglobin content in the prepared polyHb. The results demonstrate a significant reduction in methemoglobin (metHb) content upon the addition of Vitamin C, indicating its efficacy as a reducing agent. Initially, the metHb content was approximately 96.71 ± 0.67%, which dropped to 5.12 ± 1.67% within 24 hours. This sharp decline shows the potent antioxidant properties of Vitamin C in converting metHb back to oxyhemoglobin (oxyHb) [16]. OxyHb is the form of hemoglobin that binds and delivers oxygen to tissues and on the other hand, metHb is the oxidized form of hemoglobin. In metHb, the iron is in the ferric state (Fe3+) which cannot bind oxygen. As a result, metHb cannot transport oxygen. Furthermore, methemoglobin can also contribute to oxidative stress [16]. For polyHb to work properly, it needs to stay in the oxyHb form. The rapid and substantial reduction observed suggests that Vitamin C can effectively reverse the oxidation of hemoglobin in a timely manner. As a result, polyHb used in this detailed cardiomyocyte study was mixed with Vitamin C at a final concentration of 0.05% (w/v) in order to prevent metHb formation.
Cell viability test and dosage response assay were done after 24 hours of treatment following ischemic shock to better understand dose-dependent effects. The MTT assay was used to measure cell viability and cytotoxicity. This helped determine the best concentration of polyHb + 3 neoenzymes for maximum protection against ischemic shock. The results show that polyHb + 3 neoenzymes effectively protected cell viability. Absorbance values for this group were close to the no-ischemia control group. It shows that the treatment with polyHb + 3 neoenzymes was effective in maintaining cell integrity and viability after ischemic shock. Figure 2 also shows that the polyHb + 2 neoenzymes (neoSOD + neoCAT) without the neocarbonic anhydrase was significantly less effective than polyHb + 3 neoenzymes. This highlights the importance of adding neoCarbonic anhydrase for optimal recovery. The dosage response curve in Figure 3 shows that 1.4x polyHb + 3 neoenzymes gave the best cell viability. This confirms a dose-dependent effect, where increasing the concentration improves protection up to a certain point.
After that, to assess oxidative stress in ischemic cells, a ROS production assay was performed. ROS levels can change quickly after treatment. Measuring them immediately helps capture these changes and shows how effective the treatments are in reducing damage. For this reason, the cells were analyzed within 30 minutes of treatment instead of waiting for 24 hours. The results in Figures 4,5 show the effect of treatments on ROS production in 90-minute and 180-minute ischemic shock groups. In both groups, the “No Ischemia” condition had the lowest ROS levels. The Ischemic shock group (I) group had the highest ROS levels, confirming the impact of ischemia on oxidative stress. The “I + PBS” group showed no significant improvement in reducing ROS compared to I. The “I + polyHb” group slightly increased ROS levels in both cases. During ischemic conditions, oxidative stress is high, and polyHb might have increased reactive oxygen species content by introducing an influx of oxygen. This can add to the existing ROS levels in the cells. Additionally, polyHb alone does not have antioxidant enzymes to neutralize ROS, which may cause a slight increase in observed samples. Treatment with “I + 3 neoenzymes” reduced ROS significantly in both groups. The “I + polyHb + 3 neoenzymes” group had the greatest reduction in ROS, showing its strong protective effect. The “I + polyHb + 2 neoenzymes (neoSOD + neoCAT)” group also reduced ROS but less effectively than the combination with all three neoenzymes. These results suggest that combining polyHb with all three neoenzymes provides optimal protection against oxidative stress in ischemic conditions.
Next, a mitochondrial function assay was done to evaluate mitochondrial function and its recovery after ischemic shock. Mitochondria are critical in oxidative stress as they generate and respond to ROS. The Red/Green signal ratio reflects mitochondrial health, where a higher ratio indicates better function. A high ratio indicates intact mitochondrial membrane potential, reflecting healthy and functional mitochondria. A low ratio suggests mitochondrial damage, loss of membrane potential, and compromised energy production, which are key consequences of oxidative stress. In both 90-minute and 180-minute ischemic shock groups (Figures 8,9), the “No Ischemia” condition showed the highest ratio, confirming healthy mitochondria. The “Ischemic shock (I)” group had the lowest ratio, showing severe mitochondrial damage caused by ischemia. The “I + PBS” and “I + polyHb” groups showed slight improvements but still had low ratios, indicating limited recovery. The “I + 3 neoenzymes” group showed moderate recovery, which was consistent with reduced ROS levels in the ROS assay. The “I + polyHb + 3 neoenzymes” group had the best improvement in mitochondrial function, supporting its strong ROS-reducing ability. The “I + polyHb + 2 neoenzymes (neoSOD + neoCAT)” group showed moderate recovery, which aligned with the need for neocarbonic anhydrase to control intracellular pCO2. These findings confirm that combining polyHb with all three neoenzymes is the most effective treatment for restoring mitochondrial health after ischemic shock.
In addition to the mitochondrial function assay, the apoptosis and necrosis detection assay was done to evaluate whether treatments reduced cell death after ischemic shock. This study was done to determine the extent of necrosis and apoptosis and their progression following ischemia. It was analyzed after 24 hours of incubation to capture delayed effects of ischemic injury due to both ROS and increased pCO2. This allowed us to look at the long-term recovery. The Red/Green ratio was used to distinguish necrosis from early apoptosis. It would also provide insights into the severity and type of cell death. In both 90-minute and 180-minute ischemic shock groups (Figures 10,11), the “No Ischemia” condition had the lowest ratio, indicating minimal cell death. The “Ischemic shock (I)” group had the highest ratio, showing severe necrosis caused by ischemia. The “I + PBS” and “I + polyHb” groups showed moderate ratios, suggesting limited protection against cell death. The “I + 3 neoenzymes” group had a lower ratio, reflecting reduced necrosis and improved recovery. The “I + polyHb + 3 neoenzymes” group showed the lowest ratio, indicating the best reduction in apoptosis and necrosis. The “I + polyHb + 2 neoenzymes” group showed intermediate improvement. This again highlights the essential role of neoCA in mitigating ischemic shock. These results align with the mitochondrial and ROS studies. Combining polyHb with all three neoenzymes provided the most effective protection against oxidative stress and mitochondrial dysfunction.
Furthermore, the cardiac troponin I (cTnI) assay was performed to evaluate cardiac damage and recovery after ischemic shock. This study was done to determine the extent of cardiac cell damage by measuring cTnI levels. The assay was performed after 24 hours of incubation to assess the delayed effects of ischemic injury and treatment. This would have also allowed time for injury markers to accumulate in the media. Measuring cTnI levels helped evaluate long-term recovery and provided insights into cardiac injury. The “No Ischemia” group in both 90-minute and 180-minute ischemic shocks (Figures 12,13) showed the lowest cTnI levels. This result indicated minimal cardiac damage. The “Ischemic shock (I)” group had the highest levels which reflected severe cardiac injury. The “I + PBS” and “I + polyHb” groups showed moderate reductions in cTnI levels. These results indicated limited protection against cardiac damage. The “I + 3 neoenzymes” group reduced cTnI levels further which reflected reduced injury and better recovery. The “I + polyHb + 3 neoenzymes” group showed the lowest cTnI levels among all treatments. This result indicated the most effective protection against myocardial damage. The “I + polyHb + 2 neoenzymes” group showed intermediate improvement. This again shows the need for neoCarbonic anhydrase to control the intracellular pCO2.
Thus, in our study, we tested different treatments to improve myocardiacyte preservation during warm ischemia. One of the treatments used polyhemoglobin (polyHb) alone as an oxygen carrier but lacked protection against oxidative stress and cellular damage. Another treatment combined polyHb with two synthetic enzymes (neoSOD and neoCAT), which provided some antioxidant benefits but did not address all mechanisms of ischemic damage. Then, a combination of polyHb with all three synthetic enzymes was used. Each enzyme had a specific role in reducing damage during ischemia. Superoxide dismutase (SOD) reduced oxidative stress by converting superoxide radicals into hydrogen peroxide. Catalase (CAT) broke down hydrogen peroxide into water and oxygen. This in turn prevented the buildup of reactive oxygen species (ROS). Carbonic anhydrase (CA) managed the intracellular pCO2. The combination of polyHb with all three enzymes provided the best protection by targeting oxidative stress and metabolic imbalances from multiple angles. Our findings showed that this treatment improved cell viability, reduced oxidative stress, preserved mitochondrial function, minimized apoptosis and necrosis, and maintained cellular function better than polyHb combined with only two enzymes. Each enzyme targeted a specific aspect of cellular stress which created a stronger defence system against ischemia-induced damage. However, the study was conducted in an in vitro model, which may not fully replicate in vivo ischemic conditions. Moreover, the effects of polyHb + neoenzymes on microcirculation and systemic factors were not assessed in this study. Investigating the effects of polyHb + neoenzymes in an in vivo ischemia-reperfusion model would be the next step. Moreover, potential immunogenic responses to synthetic enzymes could be assessed. These approaches would help us determine if natural enzymes can be effectively replaced with these synthetic enzymes for next-generation blood substitutes.
PolyHb combined with synthetic enzymes (neoCAT, neoSOD, and neoCA) provides significant protection against ischemic injury in cardiomyocytes in vitro. The results demonstrate reduced oxidative stress, improved mitochondrial function, and decreased apoptosis and necrosis. Importantly, the inclusion of neoCA played a crucial role in enhancing cardioprotection by regulating intracellular pCO₂. These findings support the potential application of polyHb + neoenzymes in developing next-generation blood substitutes and organ preservation solutions. Further studies, particularly in in vivo models, are necessary to validate these promising results.
Data availability
All data generated or analyzed during this study are included in this published article.
Funding
Professor Chang’s laboratory is supported by the Virage Centre of Excellence in High Technology award from the Quebec Ministry of Science and Education and also by an unrestricted donation to McGill University from Pro-Heme Biotechnology.
- Chang TMS, Bülow L, Jahr JS, Sakai H, Yang C. Nanobiotherapeutic based blood substitutes. Singapore: World Scientific Publishing Co. Pte. Ltd.; 2022;1042. Available from: https://www.worldscientific.com/worldscibooks/10.1142/12054#t=aboutBook
- Chang TMS. Semipermeable microcapsules. Science. 1964;146:524–525. Available from: https://doi.org/10.1126/science.146.3643.524
- Chang TMS. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov. 2005;4:221–235. Available from: https://doi.org/10.1038/nrd1659
- Chang TMS. ARTIFICIAL CELL evolves into nanomedicine, biotherapeutics, blood substitutes, and more. Artif Cells Nanomed Biotechnol. 2019;47(1):997–1013. Available from: https://doi.org/10.1080/21691401.2019.1577885
- Chang TMS. Monograph on ‘ARTIFICIAL CELLS: biotechnology, nanotechnology, blood substitutes, regenerative medicine, bioencapsulation, cell/stem cell therapy’. Singapore/London: World Scientific Publisher/Imperial College Press. 2023;435. Available from: https://doi.org/10.3389/fmedt.2023.1306419
- Chang TMS. Stabilization of enzyme by microencapsulation with a concentrated protein solution or by crosslinking with glutaraldehyde. Biochem Biophys Res Commun. 1971;44:1531–1533. Available from: https://doi.org/10.1016/s0006-291x(71)80260-7
- Jahr J, Tseng K, Brown AP, Dubé GP. Hemoglobin-glutamer 250 (Bovine) [HBOC-201, Hemopure®] Clinical Use in South Africa and Comprehensive Review of Cardiac Outcomes and Risk/Benefit in Peer-Reviewed, Indexed Studies in Humans and Animal Models. In: Chang TMS, editor. Nanobiotherapeutic based blood substitutes. Singapore: World Scientific Publishing Co. Pte. Ltd.; 2022;207–247. Available from: http://dx.doi.org/10.1142/9789811228698_0008
- Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: The USA multicenter trial. J Am Coll Surg. 2009;208(1):1–13. Available from: https://doi.org/10.1016/j.jamcollsurg.2008.09.023
- Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299(19):2304–2312. Available from: https://doi.org/10.1001/jama.299.19.jrv80007
- Bian Y, Chang TMS. A novel nanobiotherapeutic poly-[hemoglobin-superoxide dismutase-catalase-carbonic anhydrase] with no cardiac toxicity for the resuscitation of a rat model with 90 minutes of sustained severe hemorrhagic shock with loss of 2/3 blood volume. Artif Cells Nanomed Biotechnol. 2015;43(1):1–9. Available from: https://doi.org/10.3109/21691401.2014.964554
- Bian Y, Chang TMS. Nanobiotechnological basis of an oxygen carrier with enhanced carbonic anhydrase for CO2 transport and enhanced catalase and superoxide dismutase for antioxidant function. Front Bioeng Biotechnol. 2023;11:1188399. Available from: https://doi.org/10.3389/fbioe.2023.1188399
- Hoq M, Chang TMS. Preliminary feasibility study using a solution of synthetic enzymes to replace the natural enzymes in polyhemoglobin-catalase-superoxide dismutase-carbonic anhydrase: effect on warm ischemic hepatocyte cell culture. Front Bioeng Biotechnol. 2023;11:1231384. Available from: https://doi.org/10.3389/fbioe.2023.1231384
- Hoq M, Chang TMS. Next-generation preservation solution using synthetic enzymes. Sci Rep. 2024;14:23104. Available from: https://www.nature.com/articles/s41598-024-73862-2
- Tronstad C, Pischke SE, Holhjem L, Tønnessen TI, Martinsen OG, Grimnes S. Early detection of cardiac ischemia using a conductometric PCO2 sensor: Real-time drift correction and parameterization. Physiol Meas. 2010;31:1241–1255. Available from: https://doi.org/10.1088/0967-3334/31/9/013
- Liu J. Zinc-based deep eutectic solvent – an efficient carbonic anhydrase mimic for CO2 hydration and conversion. Nat Commun. 2021;12(1):1–11.
- Chen G, Chang TMS. Dual effects include antioxidant and pro-oxidation of ascorbic acid on the redox properties of bovine hemoglobin. Artif Cells Nanomed Biotechnol. 2018;46(sup2):983–992. Available from: https://doi.org/10.1080/21691401.2018.1476374