Figure 1
Thrombolysis, the only Optimally Rapid Reperfusion Treatment
Victor Gurewich*
Published: 23 June, 2017 | Volume 2 - Issue 1 | Pages: 029-034
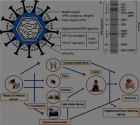
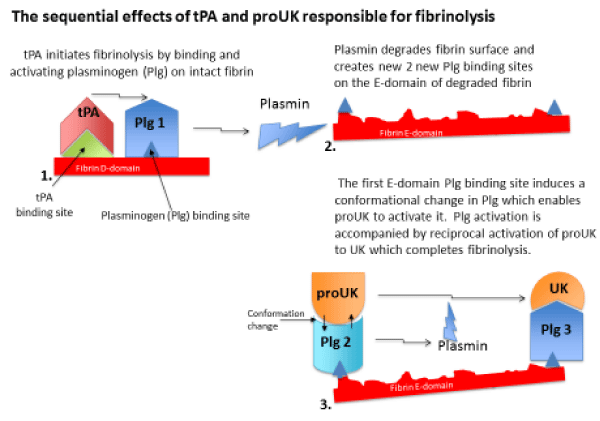 Fibrinolysis is initiated when tPA released from the vessel wall binds to its binding site on the D-domain of intact fibrin and activates plasminogen bound to an adjacent site. This ternary complex with fibrin promotes tPA plasminogen activation by about 1,000-fold. Fibrin degradation creates two new plasminogen binding sites on the fibrin E-domain. The fi rst of these is a triple carboxyl lysine binding site which induces a unique conformational
Fibrinolysis is initiated when tPA released from the vessel wall binds to its binding site on the D-domain of intact fibrin and activates plasminogen bound to an adjacent site. This ternary complex with fibrin promotes tPA plasminogen activation by about 1,000-fold. Fibrin degradation creates two new plasminogen binding sites on the fibrin E-domain. The fi rst of these is a triple carboxyl lysine binding site which induces a unique conformational change in plasminogen that enables the intrinsic activity of proUK to activate it. This is accompanied by reciprocal activation of proUK to UK which activates the remaining plasminogen completing fibrinolysis." alt="jccm-aid1010-g001"class="img-responsive img-rounded " style="cursor:pointer">
Figure 1:
Fibrinolysis is initiated when tPA released from the vessel wall binds to its binding site on the D-domain of intact fibrin and activates plasminogen bound to an adjacent site. This ternary complex with fibrin promotes tPA plasminogen activation by about 1,000-fold. Fibrin degradation creates two new plasminogen binding sites on the fibrin E-domain. The fi rst of these is a triple carboxyl lysine binding site which induces a unique conformational
change in plasminogen that enables the intrinsic activity of proUK to activate it. This is accompanied by reciprocal activation of proUK to UK which activates the remaining plasminogen completing fibrinolysis.
Read Full Article HTML DOI: 10.29328/journal.jccm.1001010 Cite this Article Read Full Article PDF
More Images
Similar Articles
-
Left Atrial Remodeling is Associated with Left Ventricular Remodeling in Patients with Reperfused Acute Myocardial InfarctionChristodoulos E. Papadopoulos*,Dimitrios G. Zioutas,Panagiotis Charalambidis,Aristi Boulbou,Konstantinos Triantafyllou,Konstantinos Baltoumas,Haralambos I. Karvounis,Vassilios Vassilikos. Left Atrial Remodeling is Associated with Left Ventricular Remodeling in Patients with Reperfused Acute Myocardial Infarction. . 2016 doi: 10.29328/journal.jccm.1001001; 1: 001-008
-
Mid-Ventricular Ballooning in Atherosclerotic and Non-Atherosclerotic Abnormalities of the Left Anterior Descending Coronary ArteryStefan Peters*. Mid-Ventricular Ballooning in Atherosclerotic and Non-Atherosclerotic Abnormalities of the Left Anterior Descending Coronary Artery. . 2016 doi: 10.29328/journal.jccm.1001002; 1:
-
Concentration Polarization of Ox-LDL and Its Effect on Cell Proliferation and Apoptosis in Human Endothelial CellsShijie Liu*,Jawahar L Mehta,Yubo Fan,Xiaoyan Deng,Zufeng Ding*. Concentration Polarization of Ox-LDL and Its Effect on Cell Proliferation and Apoptosis in Human Endothelial Cells. . 2016 doi: 10.29328/journal.jccm.1001003; 1:
-
Intermittent Left Bundle Branch Block: What is the Mechanism?Hussam Ali*,Riccardo Cappato. Intermittent Left Bundle Branch Block: What is the Mechanism?. . 2017 doi: 10.29328/journal.jccm.1001004; 2:
-
Congenital Quadricuspid Aortic Valve, a Rare Cause of Aortic Insufficiency in Adults: Case ReportCyrus Kocherla*,Kalgi Modi. Congenital Quadricuspid Aortic Valve, a Rare Cause of Aortic Insufficiency in Adults: Case Report. . 2017 doi: 10.29328/journal.jccm.1001005; 2: 003-007
-
Short and Medium-Term Evaluation of Patients in Coronary Post-Angioplasty: Préliminary results at the Cardiology Department of the Hospital University Aristide Le Dantec of Dakar (Senegal): Study on 38 CasesDioum M*,Aw F,Masmoudi K,Gaye ND,Sarr SA,Ndao SCT, Mingou J,Ngaidé AA,Diack B,Bodian M,Ndiaye MB,Diao M,Ba SA. Short and Medium-Term Evaluation of Patients in Coronary Post-Angioplasty: Préliminary results at the Cardiology Department of the Hospital University Aristide Le Dantec of Dakar (Senegal): Study on 38 Cases. . 2017 doi: 10.29328/journal.jccm.1001006; 2: 008-012
-
Indications and Results of Coronarography in Senegalese Diabetic Patients: About 45 CasesNdao SCT*,Gaye ND,Dioum M,Ngaide AA,Mingou JS,Ndiaye MB, Diao M,Ba SA. Indications and Results of Coronarography in Senegalese Diabetic Patients: About 45 Cases. . 2017 doi: 10.29328/journal.jccm.1001007; 2: 013-019
-
Procedure utilization, latency and mortality: Weekend versus Weekday admission for Myocardial InfarctionNader Makki,David M Kline,Arun Kanmanthareddy,Hansie Mathelier,Satya Shreenivas,Scott M Lilly*. Procedure utilization, latency and mortality: Weekend versus Weekday admission for Myocardial Infarction. . 2017 doi: 10.29328/journal.jccm.1001008; 2: 020-025
-
Spontaneous rupture of a giant Coronary Artery Aneurysm after acute Myocardial InfarctionOğuzhan Çelik,Mucahit Yetim,Tolga Doğan,Lütfü Bekar,Macit Kalçık*,Yusuf Karavelioğlu. Spontaneous rupture of a giant Coronary Artery Aneurysm after acute Myocardial Infarction. . 2017 doi: 10.29328/journal.jccm.1001009; 2: 026-028
-
Thrombolysis, the only Optimally Rapid Reperfusion TreatmentVictor Gurewich*. Thrombolysis, the only Optimally Rapid Reperfusion Treatment. . 2017 doi: 10.29328/journal.jccm.1001010; 2: 029-034
Recently Viewed
-
The Risk-Adjusted Impact of Intraoperative Hemofiltration on Real-World Outcomes of Patients Undergoing Cardiac SurgeryMatata BM*,Shaw M. The Risk-Adjusted Impact of Intraoperative Hemofiltration on Real-World Outcomes of Patients Undergoing Cardiac Surgery. J Clini Nephrol. 2017: doi: 10.29328/journal.jcn.1001001; 1: 001-010
-
Stroke Mimics: Insights from a Retrospective Neuroimaging StudyLucia Monti*, Davide del Roscio, Francesca Tutino, Tommaso Casseri, Umberto Arrigucci, Matteo Bellini, Maurizio Acampa, Sabina Bartalini, Carla Battisti, Giovanni Bova, Alessandro Rossi. Stroke Mimics: Insights from a Retrospective Neuroimaging Study. J Neurosci Neurol Disord. 2023: doi: 10.29328/journal.jnnd.1001083; 7: 094-103
-
Estimating the Minimum Possible Deceleration of Cosmic Expansion Post-inflationDev Sharma*. Estimating the Minimum Possible Deceleration of Cosmic Expansion Post-inflation. Int J Phys Res Appl. 2024: doi: 10.29328/journal.ijpra.1001089; 7: 080-085
-
Giant cellular angiofibroma of the vulva: case reportAna Ribeiro*,Isabel Ferreira,Filomena Ramos. Giant cellular angiofibroma of the vulva: case report. Clin J Obstet Gynecol. 2019: doi: 10.29328/journal.cjog.1001016; 2: 003-005
-
Coronavirus and pHViktor Zinchenko* and Adriana Barylyak. Coronavirus and pH. Int J Clin Virol. 2023: doi: 10.29328/journal.ijcv.1001052; 7: 003-006
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."