More Information
Submitted: May 26, 2022 | Approved: June 06, 2022 | Published: June 07, 2022
How to cite this article: Yadav V, Thapa S, Gajurel RM, Poudel CM, Manandhar B, et al. A Wolff-Parkinson-White (WPW) Electrocardiographic Pattern in Asymptomatic Patient – State-of-the-Art-Review. J Cardiol Cardiovasc Med. 2022; 7: 046-053.
DOI: 10.29328/journal.jccm.1001132
Copyright License: © 2022 Yadav V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: WPW pattern; Electrophysiology study; Catheter ablation
A Wolff-Parkinson-White (WPW) Electrocardiographic Pattern in Asymptomatic Patient – State-of-the-Art-Review
Vijay Yadav1* , Sanjeev Thapa1, Ratna Mani Gajurel1, Chandra Mani Poudel1, Bhawani Manandhar1, Manju Sharma1, Suman Adhikari2 and Suraj Shrestha3
, Sanjeev Thapa1, Ratna Mani Gajurel1, Chandra Mani Poudel1, Bhawani Manandhar1, Manju Sharma1, Suman Adhikari2 and Suraj Shrestha3
1Department of Cardiology, Manmohan Cardiothoracic Vascular and Transplant Center (MCVTC), Institute of Medicine (IOM), Kathmandu, Nepal
2Department of Cardiology, Pokhara Academy of Health Sciences (PAHS), Pokhara, Nepal
3MD Candidate, Maharajgunj Medical Campus, Institute of Medicine (IOM), Kathmandu, Nepal
*Address for Correspondence: Dr. Vijay Yadav, Department of Cardiology, Manmohan Cardiothoracic Vascular and Transplant Center (MCVTC), Institute of Medicine (IOM), Kathmandu, Nepal, Email: vjmedicine451@gmail.com
A comprehensive approach to asymptomatic adults with Wolff-Parkinson-White (WPW) pattern discovered incidentally on routine electrocardiography (ECG) is debatable. The objective of this review article is to update the most recent evidence on the management of young patients with asymptomatic WPW patterns. A substantial proportion of adults with WPW patterns on ECG may remain asymptomatic but the lifetime risk for fatal arrhythmias still exists. The inherent properties of the accessory pathway determine the risk of sudden cardiac death. A low-risk pathway is considered when the pre-excitation is intermittent on ambulatory monitoring or when it disappears completely or abruptly during exercise testing. On the other hand, a high-risk pathway in EP study is suggested by the presence of the shortest pre-excited RR interval (SPERRI) during atrial fibrillation of ≤ 250 ms or accessory pathway effective refractory period (APERP) ≤ 240 ms. The cardiac evaluation may thus be considered in asymptomatic patients with WPW to determine the individual risk for future symptomatic arrhythmia. A shared-decision making must be performed before offering catheter ablation whose procedural success rate is high.
In 1921, a phenomenon of “intraventricular block and a PR interval of 0.08 ms” in a 19-year-old patient with paroxysms of tachycardia was first described by A.M. Wedd [1]. It was in 1930, when Louis Wolff, John Parkinson, and Paul D. White together described a set of eleven cases of an electrocardiographic pattern consisting of a “functional bundle branch” and a “short PR interval” in the healthy young people with paroxysms of tachycardia [2]. However, it is not uncommon to incidentally find a Wolff-Parkinson-White (WPW) pattern in a routinely performed electrocardiography (ECG) without any arrhythmia. WPW pattern is a ventricular pre-excitation wherein an accessory bypass tract known as the bundle of Kent serves as the connection between the atrial to the ventricular myocardium bypassing the atrioventricular (AV) node. When electrical impulses are conducted via this accessory tract, it leads to premature ventricular activation described as pre-excitation [3].
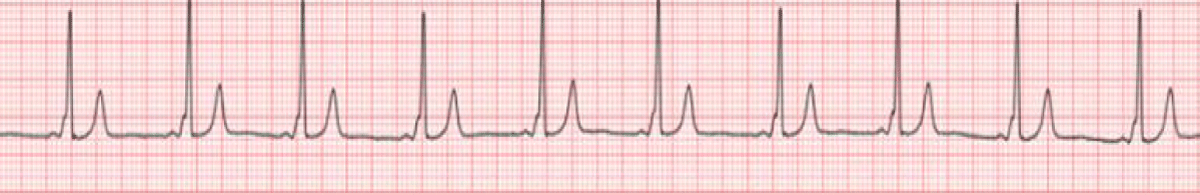
The WPW syndrome affects 0.1-0.3% of the general population [4]. The characteristic ECG features are (i) shortened PR interval (< 120 ms) due to early ventricular depolarization that results due to bypassing of the AV nodal delay, (ii) slurred QRS upstroke (delta wave) that occurs due to ventricular pre-excitation, and (iii) prolonged QRS duration (> 120 ms) as it is formed by the sum of normal ventricular activation and ventricular pre-excitation through the accessory tract. In the absence of a documented tachyarrhythmia or related symptoms, the ECG findings alone are referred to as the WPW pattern [5]. Figure 1 shows the characteristic WPW pattern in the ECG.
Figure 1: Sinus rhythm with typical WPW pre-excitation pattern manifesting as a short PR interval, delta wave, and wide QRS complex [6].
The purpose of this review article is to discuss the comprehensive approach to an asymptomatic patient with a WPW pattern in ECG and also to highlight a few salient features of different accessory pathways whenever feasible.
Anatomy of the accessory pathways
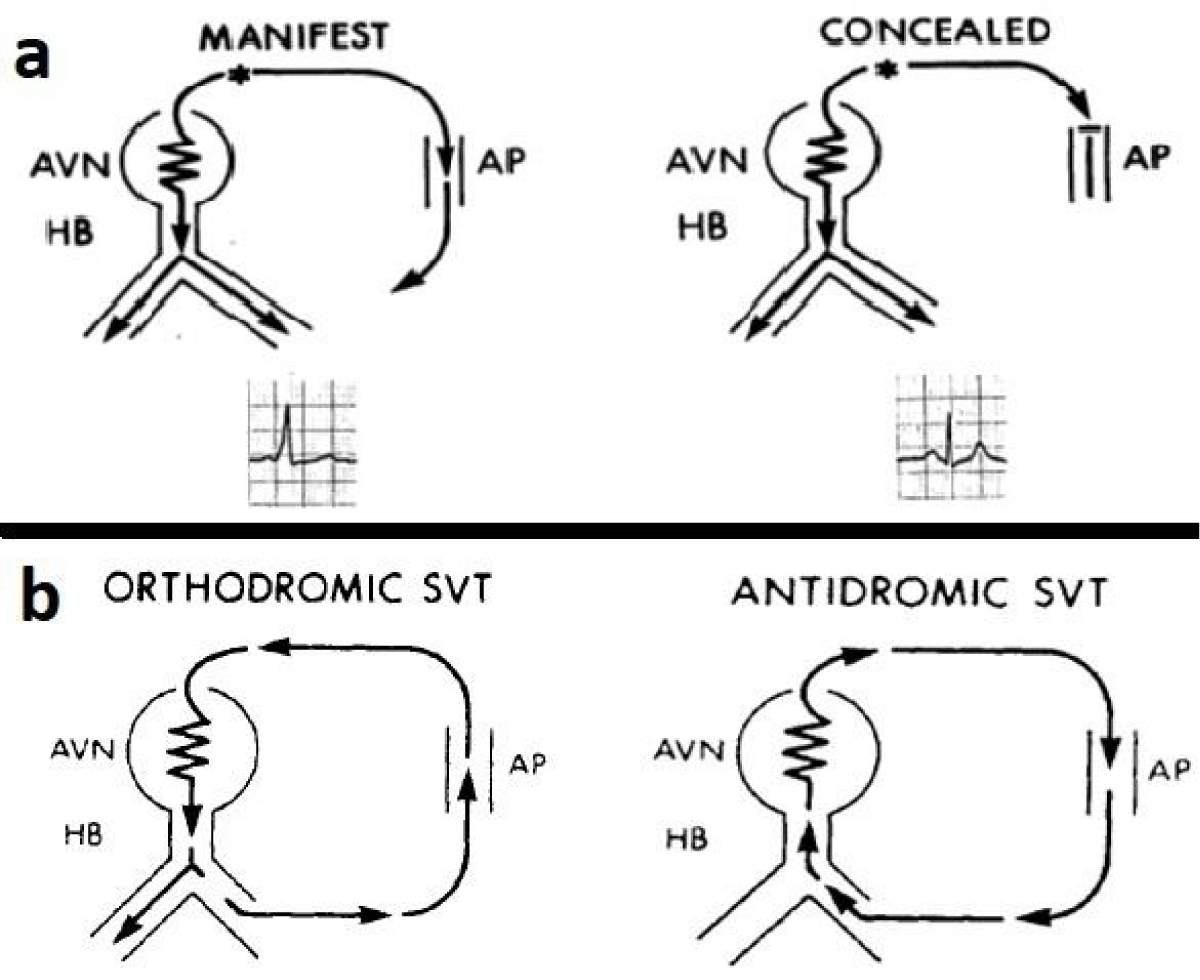
The accessory pathway (AP), which leads to pre-excitation, results from a developmental failure to eradicate the remnants of the atrioventricular connections present during cardiogenesis. It also results from the anomalous myocardial tissue spanning the fibrinous bridges between the atria and ventricles [2]. Morphologically, accessory pathways are the strands of normal myocardium that bridge the AV groove at any point around the annulus fibrosus on either side of the heart except that portion of the mitral valve annulus between the right and left fibrous trigones [7]. The locations of the accessory pathways are regionalized to the left-free wall (58%), posterior septal (24%), right free-wall (13%), and anterior septal (5%) sites, respectively [8]. The accessory pathways usually exhibit rapid and non-decremental conduction and have a longer effective refractory period (ERP) than that of the AV node. The atrial and ventricular insertions of accessory pathways (free-wall APs) are located between the valve annulus and the atrial and ventricular epicardial reflections, respectively [9]. The posterior septal pathways are located within the pyramidal space, bounded anteriorly by the insertion of the atrial extension of the membranous septum into the right fibrous trigone and posteriorly by the epicardium overlying the crux of the heart. The lateral boundaries are formed by divergent walls of the left and right atria. Within this space is the AV nodal artery, the tendon of Todaro, epicardial fat, and the proximal portion of the coronary sinus. The AV node and its proximal penetrating bundle lie within the triangle of Koch, immediately adjacent to the pyramidal space. The anterior septal pathways are located just anterior to the AV node and pass through the fat pad between the right and left fibrous trigones and the insertion of the right coronary artery into the AV groove. This path lies anterior to the membranous portion of the interatrial septum and is bounded by the pericardial reflection of the ascending aorta and medial wall of the right atrium [10]. The accessory pathway present in the WPW pattern is capable of conducting in both antegrade and retrograde directions that eventually begets a re-entrant supraventricular tachycardia (SVT) [11]. The orthodromic SVT, in which the anterograde conduction to the ventricles is through the AV node/His bundle, and the retrograde conduction to the atria is through the accessory pathway, accounts for 90% of arrhythmia. This is also known as a concealed accessory pathway in which there is no pre-excitation in the ECG but the patients are prone to develop orthodromic SVT. The antidromic SVT occurs in 10% of patients with the WPW syndrome in which the anterograde limb is the accessory pathway, and the retrograde limb is either the normal conduction system or a second accessory pathway. This is also known as a manifest accessory pathway in which there is pre-excitation in the ECG and the patients are prone to develop antidromic SVT [10]. This is shown in Figure 2.
Figure 2: The concealed and manifest pathways (a-upper row) and schematic representations of orthodromic and antidromic SVTs (b- lower row) [8].
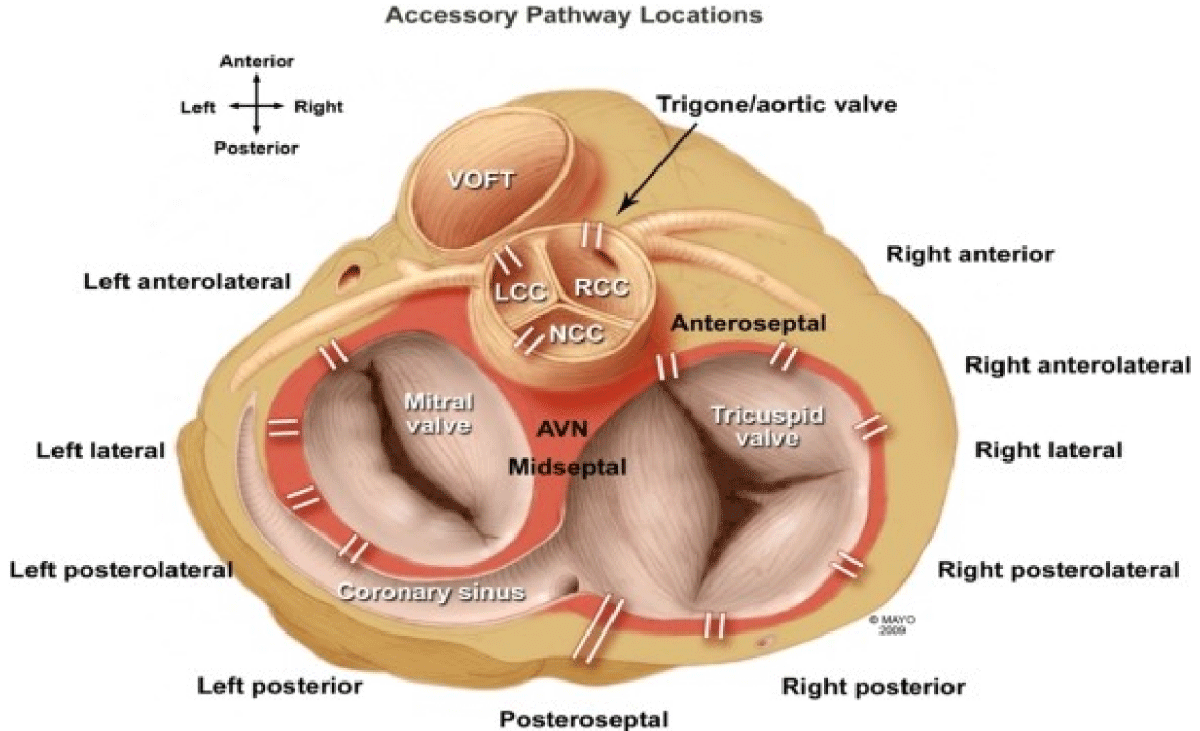
It is a well-known fact that the accessory pathway allows for rapid ventricular conduction in the presence of atrial fibrillation, which, in turn, might degenerate into ventricular fibrillation with the hemodynamic collapse that leads to sudden cardiac death (SCD). Figure 3 [12] depicts a detailed anatomical location of various accessory pathways.
Figure 3: Accessory pathways location.
The characteristics of various accessory pathways are depicted in Table 1.
| Table 1: Types of accessory pathways and their ECG features. | ||
| Pathways | PR interval | Delta wave |
| Atrioventricular (Kent) | Short | Present |
| Atrionodal (James) | Short | Absent |
| Atriohisian (Brechenmaker) | Short | Absent |
| Atriofascicular (Mahaim) | Normal | Present |
| Nodofascicular (Mahaim) | Normal | Present |
| Fasciculoventricular | Normal | Present |
| Nodoventricular | Normal/Decreased | Present |
Atriofascicular and Nodofascicular (Mahaim) connections represent a duplication of the AV node-His Purkinje system along the tricuspid annulus. It is typically a right-sided pathway that inserts apically into the distal right bundle branch or moderator band. These connections always conduct anterogradely with a left bundle branch block (LBBB)-like-pre-excited QRS morphology with atrial extra stimuli, atrial pacing, and tachycardia. The pathway has anterograde decremental (AV-node-like) properties and is adenosine sensitive. Adenosine administration during tachycardia usually results in sudden termination of the tachycardia by anterograde block in the atriofascicular pathway [13]. The occurrence of Mahaim fibers is unusual, comprising < 3% of accessory pathways [14].
Conduction through a typical AP is not affected by adenosine. In contrast, accessory pathways like the atriofascicular and retrograde posteroseptal pathway of permanent junctional reciprocating tachycardia with a long conduction time (decremental conducting) can be blocked by adenosine [15]. The fasciculoventricular pathway is a rare variant that arises from his bundle or bundle branch and inserts into the ventricle. It does not usually require ablation owing to the fact that clinical arrhythmias are rare with such AP. Nodoventricular and Nodofascicular pathways are also rare and they bypass a portion of the AV node and insert directly into the crest of the ventricular septum and conduction system, respectively. Because the AV node has decremental conduction, these pathways are also sensitive to adenosine [16].
Clinical presentation
In the absence of any symptoms, a WPW pattern may be an incidental finding in the routinely performed ECG. In those patients who are symptomatic, arrhythmias, predominantly in the form of AV re-entrant tachycardia (AVRT) and atrial fibrillation (AF) are the commonest presentations. AVRT is the most common arrhythmia accounting for 95% of re-entrant tachycardias and AF has been estimated to be present in one-third of cases of WPW syndrome. Ventricular fibrillation is the dominant cause of SCD in patients with WPW syndrome [17]. Commonly, there is anterograde conduction through the AV node that returns retrogradely via a bypass tract to the atria leading to an orthodromic tachycardia that would result in narrow QRS complexes. Less commonly, there occurs anterograde conduction through the bypass tract, returning retrogradely to the atria via the normal AV nodal pathway leading to antidromic tachycardia resulting in wide QRS complexes [18].
Localisation of accessory pathway in the ECG
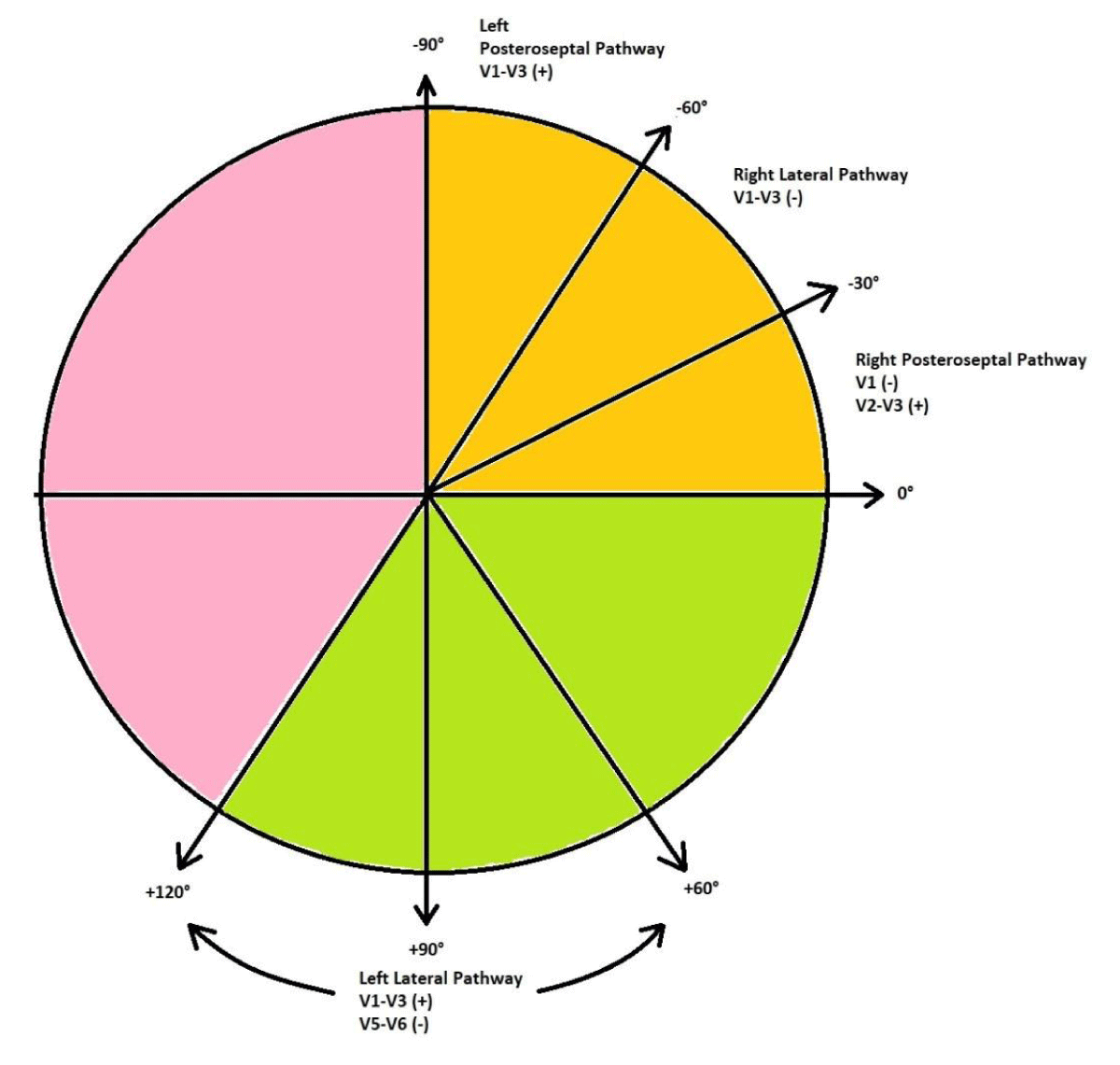
The pre-excited 12-lead ECG provides information as to the likely site of the accessory pathway, and several algorithms have been developed and validated. We present a simple and concise method of localizing the accessory pathway in the ECG as shown in Table 2 [19] and Figure 4.
| Table 2: ECG localization of accessory pathways. | ||||
| I, aVL | II, III, aVF | QRS axis | Precordial leads (QRS polarity) |
Pathway |
| Positive | Negative | 0◦ to -30◦ | V1: Negative V2-V3: Positive |
Right Posteroseptal |
| Positive | Negative | -30◦ to -60◦ | V1-V3: Negative | Right Lateral |
| Positive | Negative | -60◦ to -90◦ | V1 –V3: Positive | Left Posteroseptal |
| Negative | Positive | +90◦ to +120◦ | V1-V3: Positive V5-V6: Negative |
Left Lateral |
| Positive | Positive | Normal | V1-V3: Negative | Anteroseptal |
Figure 4: A simplified diagram to localize accessory pathways.
Types of WPW pattern
The WPW pattern can have three QRS morphologies based on the direction of the accessory pathway. The various types are shown in Table 3 [20].
| Table 3: Types of WPW patterns. | |||
| Types | Connection | Precordial leads (QRS polarity) |
Can mimic |
| Type A WPW | Left septal connection | V1-V6: Positive | RBBB or Posterior wall MI |
| Type B WPW | Right-sided connection | V1: Negative V6: Positive |
LBBB or LVH |
| Type C WPW | Left lateral connection | V1: Positive V6: Negative |
RVH |
| RBBB: Right Bundle Branch Block; LBBB: Left Bundle Branch Block; LVH: Left Ventricular Hypertrophy; RVH: Right Ventricular Hypertrophy. | |||
Natural history of WPW pattern
The prevalence of WPW is estimated to be 1-3 in 1000 individuals based on large-scale population-based studies [21]. Among the first-degree relatives following an index case of WPW, the incidence was found to be 5.5 in 1000 individuals [22]. On a resting ECG with a WPW pattern, approximately 65% of adolescents and 40% of adults over 30 years are asymptomatic [23]. The majority of patients with WPW patterns have a structurally normal heart. However, it can occur commonly in the presence of Ebstein’s anomaly and hypertrophic cardiomyopathy, and uncommonly in the presence of cardiac rhabdomyoma [5]. In symptomatic patients with palpitations and pre-syncope, arrhythmias in the form of atrioventricular reciprocating tachycardia (AVRT) and atrial fibrillation (AF) are most commonly encountered. Rapid conduction of AF over the accessory pathway resulting in ventricular fibrillation (VF) is rare but unfortunately may be the first manifestation of WPW syndrome [24]. Various studies have estimated the overall lifetime risk of SCD in asymptomatic WPW patients to be 3% - 4%, with the most cases between ages 10 and 40 years [25] and SCD has been found to be the first presentation in 65% of patients with asymptomatic WPW pattern [26]. Of note, asymptomatic WPW patterns are also being increasingly identified in children during routine screening and investigations for an unrelated illness. The risk of SCD in these subjects is significantly increased compared to children without WPW. Ablation of AP is deemed necessary in children if SPERRI-AF is less than 250 ms whereas asymptomatic WPW in children requires careful and informed management on a case-by-case basis. [27]. Hence, the accurate identification of high-risk features for SCD can help prevent this dreaded outcome.
Risk stratification in an asymptomatic WPW pattern
The main purpose of risk stratification in asymptomatic patients with a WPW pattern is to identify which individuals are at an increased risk of lethal arrhythmia and SCD.
Patient history: On clinical evaluation, the high-risk features include male sex, familial WPW syndrome (autosomal dominant, chromosome 7, PRKAG2 gene mutation), WPW pattern detected in the first two decades of life, history of atrial fibrillation and arrhythmic symptoms like syncope, and presence of congenital heart disease, especially, Ebstein’s anomaly. Also, high-risk occupations such as those of pilots, bus drivers, and athletes should be given a special priority [4].
Non-invasive testing: In asymptomatic patients with WPW patterns, non-invasive tests like 12-lead ECG, ambulatory ECG monitoring, and exercise stress test (EST) are considered safe and should be done [28].
The intermittent loss of pre-excitation during sinus rhythm on serial ECGs or ambulatory monitoring confers a low risk for cardiac arrest because it signifies poor anterograde conduction through the accessory pathway owing to the long ERP of the AP (APERP) [29]. An earlier study conducted by Hindman, et al. [30] showed that the incidence of intermittent pre-excitation in ambulatory ECG monitoring was 67%. Patients with intermittent pre-excitation are less prone to develop SVT in comparison to patients with constant pre-excitation. In a large study of primarily, military aviators followed over 2 decades, 23% with constant preexcitation developed reentrant SVT in comparison to the 8.3% who only exhibited intermittent preexcitation [31]. The appearance of different pre-excited morphologies on the ECG or ambulatory monitoring denotes a higher risk for SCD [32]. In asymptomatic patients who are not cognizant of brief atrial arrhythmia, ambulatory ECG monitoring should be done to screen for paroxysmal AF and the detection rate of Holter monitoring for paroxysmal AF is 12% as evidenced by the long-term prospective study conducted by Santinelli, et al. [33].
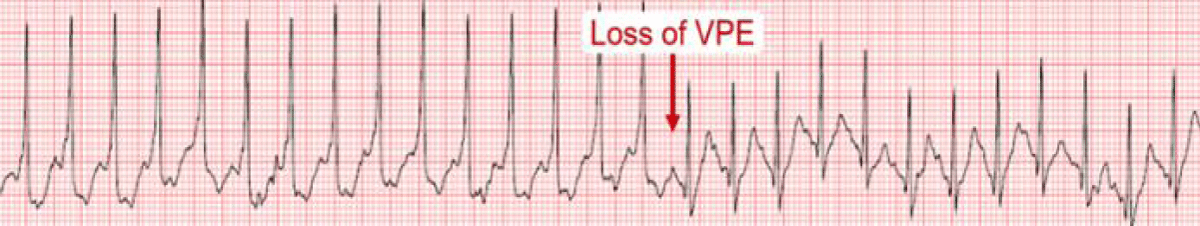
The effect of sympathetic stimulation on the accessory pathway refractoriness and AV nodal conduction affects the delta wave behavior during exercise. The exercise-induced rapid AV nodal conduction may mask persistent pre-excitation (Figure 5) and portends a low risk for VF. However, the frequency of anterograde conduction block over AP is only 15% and, hence, it is a low sensitivity finding and it does not exclude high-risk accessory Aps [34]. The APERP can be indirectly assessed by noting the disappearance of pre-excitation during exercise testing [35]. Persistence of pre-excitation during EST has a sensitivity of 96%, specificity of 17%, a positive predictive value of 40%, and a negative predictive value of 88%, respectively in predicting APERP ≤ 250 milliseconds (ms) [36].
Figure 5: Exercise-induced loss of pre-excitation [6].
Pharmacological challenge test: The sodium channel blocking agents have been used in the past to determine the properties of an accessory pathway - although no longer utilized nowadays. Block of conduction in the accessory pathway after Ajmaline [37] or Procainamide [38] infusion is known to have been associated with long APERP and, thus, could be used to identify patients unlikely to be at risk for sudden death in the event of atrial fibrillation.
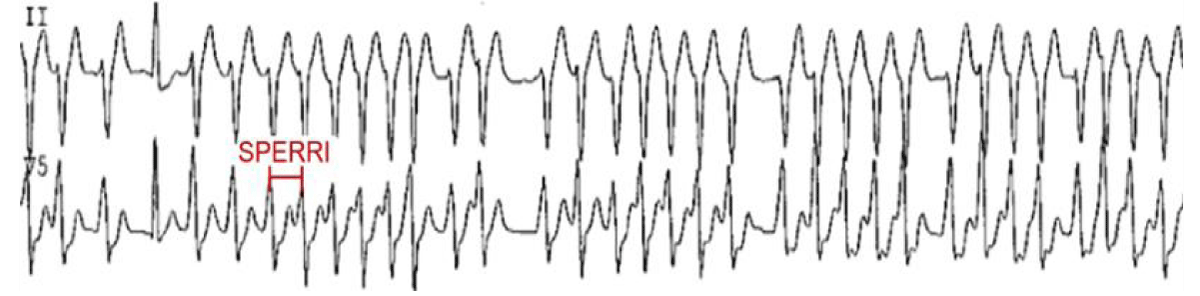
Invasive Electrophysiological (EP) testing: When the non-invasive tests are inconclusive regarding the anterograde conduction of AP, an invasive EP study should be considered. The key measurements include APERP and shortest pre-excited RR interval (SPERRI) during AF. The APERP represents the longest coupling interval that cannot conduct over the AP. The longer the APERP, the lower the risk of fatal arrhythmia induction. The SPERRI, which is measured during spontaneous or induced AF, represents the shortest time between pre-excited QRS complexes during AF. The short APERP and SPERRI suggest rapid anterograde conduction and are associated with a high-risk WPW [5]. Baseline measurements of APERP ≤ 240 ms and SPERRI ≤ 250 ms during AF are considered high-risk features (Figure 6). The electrophysiological properties in asymptomatic adults with WPW pattern that render them low risk for SCD are consequent loss of their capacity for anterograde conduction (in 20% of patients) [39] and the lack of retrograde conduction through the AP during EP testing (in 27% of patients) [40]. Despite this potentially favorable natural history in adults, some asymptomatic adults with WPW may have EP indices that harbor concern for the possibility of SCD in the future. A SPERRI < 250 ms has been found to be in 20%-26% of patients with asymptomatic WPW patterns [41].
Figure 6: SPERRI measurement during rapid pre-excited AF that can potentially precipitate ventricular fibrillation [6].
The SPERRI measurement appears superior to APERP measurement in discriminating patients at risk for SCD and who may eventually benefit from ablation [42]. In 1979, Klein and his colleagues [24] published a detailed electrophysiological assessment of 25 patients with WPW and documented VF and compared them to 73 patients without VF. They noted that the SPERRI-AF was the most useful discriminator, with a cutoff of 250 ms having 100% sensitivity for the VF group, albeit with a specificity of only 35%. In yet another similar study [5], the sensitivity and negative predictive value of SPERRI ≤ 250 ms were shown to be high and the specificity and positive predictive value were found to be low in identifying risk in patients with symptomatic WPW but the specificity and positive predictive value were even lower in asymptomatic patients because the incidence of subsequent SCD in them was low.
These basic measurements during an EP study can be performed by isoproterenol infusion, programmed atrial stimulation, and both atrial and ventricular stimulation. By serving as a possible surrogate for physiologic adrenergic stimulation and enhancing AP conduction, the isoproterenol infusion during EP testing has been proposed to identify high-risk patients [43]. But the major fallacy of isoproterenol infusion is that it can result in APERP fluctuations secondary to the autonomic system modifications of the AP and AVN. It was shown in a study of 21 asymptomatic adults with WPW pattern [44] that isoproterenol administration significantly shortened the SPERRI from 264 to 219 ms which further depicted a total of 67% of patients had a SPERRI ≤ 250 ms on isoproterenol compared to 33% at baseline. Thus, APERP measurement during isoproterenol use is less than reliable in risk stratification in asymptomatic WPW patients.
The APERP and SPERRI can also be evaluated utilizing a programmed atrial stimulation at high rates near the AP atrial insertion site which can determine the maximal rate at which 1:1 AV conduction occurs over the AP. To measure SPERRI, induction of AF can be attempted. The evaluation of rapid conduction should rely on APERP or 1:1 AV conduction over AP if AF cannot be induced. Spontaneous degeneration into pre-excited AF upon induction of AVRT is considered a high-risk feature [6].
With a focus on finding out the exact numbers and locations of the accessory pathways, atrial and ventricular stimulation is widely performed [5]. The risk of arrhythmic events and subsequent SCD is increased in the presence of a septal location of AP or with the presence of multiple Aps [42]. In symptomatic patients, the presence of multiple APs and septal bypass tracts may be a separate risk factor for the development of VF [24]. The lower risk features include lack of tachyarrhythmia inducibility, lack of retrograde AP conduction, and absence of multiple Aps [25].
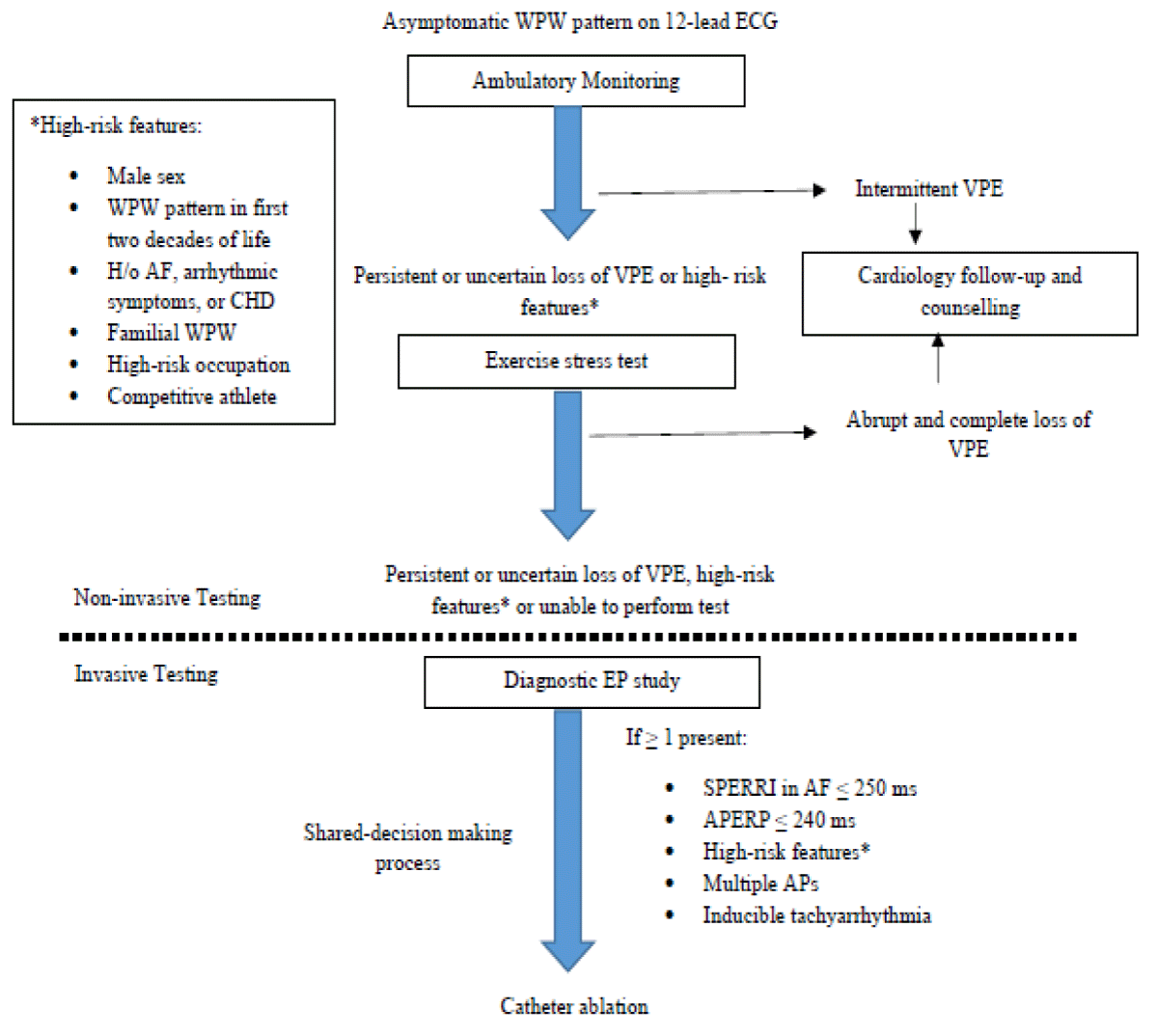
In Figure 7, we present a simplified algorithm regarding the accepted approach to asymptomatic adults with WPW patterns in the ECG.
Figure 7: A Simplified approach to asymptomatic WPW evaluation [6].
Management of the asymptomatic patients
Pharmacological therapy: There is no proven pharma-cological therapy for asymptomatic patients and, hence, it is not recommended [45].
Catheter ablation: Radiofrequency ablation (RFA) was first introduced in 1990 as a definitive cure for arrhythmias in the pediatric population with WPW syndrome and is now widely accepted as first-line therapy due to its high success rate and low-risk profile [5]. However, an acrimonious dispute remains as to when and whether ablation should be performed in asymptomatic WPW patients. The scathing question that needs to be answered is whether the calculated benefit of catheter ablation outweighs the risks of the procedure. The consensus is that if SPERRI during AF is ≤ 250 ms and APERP during programmed atrial stimulation is ≤ 240 ms, if an AP contributes to clinical arrhythmia, if any high-risk feature is present, or if there are multiple APs, then prophylactic catheter ablation of the accessory pathway is recommended [46]. The reported success rate of catheter ablation is over 90% and the complication rate is under 2%. The major complications include infection, bleeding, heart block, cardiac tamponade, thromboembolic events, and cerebrovascular accidents [45].
A zero fluoroscopy method can be applied nowadays for both left and right-sided ablation procedures. Newer 3-dimensional mapping technologies minimize fluoroscopy use. The important benefits of the zero-fluoroscopy approach are decreased radiation exposure, staff comfort, and easy applicability for pregnant females and young females who wish to conceive in the future. In an earlier study conducted by Bigelow, et al. it was concluded that the zero-fluoroscopy approach with the 3-dimensional mapping system using the Ensite system virtually eliminated fluoroscopy use in routine ablation procedures [47]. Zero and near-zero fluoroscopic ablation of cardiac arrhythmias, which is also known as “green electrophysiology”, is gradually being introduced into the clinical practice. A variety of measures including the use of a remote magnetic navigation system, new Three-Dimensional Electro-Anatomical Mapping (3D-EAM) software, Intracardiac Echocardiography (ICE), miniaturized Transesophageal Echocardiography (TEE), and contact-force sensing catheters, have led to a significant reduction or complete elimination of fluoroscopy in most patients, particularly in children, pregnant women, and patients with immune dysfunction [48].
The recommended management guidelines according to the American College of Cardiology/American Heart Association (ACC/AHA) for asymptomatic adults with a WPW pattern in ECG are summarized in Table 4 [4].
| Table 4: Asymptomatic Patients With Pre-Excitation: Recommendations. | |
| Class of recommendations | Recommendations |
| Class I | Abrupt loss of conduction over a manifest pathway during EST in sinus rhythm, intermittent loss of preexcitation during ECG, and ambulatory monitoring are all useful tests in order to identify patients at low risk of SCD. |
| Class IIa | An EP study is reasonable to risk-stratify for arrhythmic events. |
| Catheter ablation of the accessory pathway is reasonable if an EP study identifies a high risk of arrhythmic events, including rapidly conducting pre-excited AF. | |
| Catheter ablation of the accessory pathway is reasonable in asymptomatic patients if the presence of pre-excitation precludes specific employment (such as with pilots). | |
| Observation, without further evaluation or treatment, is reasonable in asymptomatic patients with pre-excitation. | |
Association between asymptomatic Wolff-Parkinson-White (WPW) syndrome and sudden cardiac death (SCD) has been well documented. Such asymptomatic patients should be subjected to different non-invasive and invasive tests to classify them as low or high risk for SCD. Regular cardiac follow-up, reassurance, and proper counseling are the mainstay of therapy for low-risk patients whereas radiofrequency ablation (RFA) of the accessory pathway is the definitive therapy for high-risk patients. Further studies are needed to identify predictors for future cardiac events and to assess whether medical intervention can reduce overall risk.
Grateful acknowledgments are due to Prof. Dr. Ramawatar Yadav for his abiding inspiration, expert advice and suggestions, and professional support towards the completion of this research work.
- WEDD AM. Paroxysmal tachycardia: With reference to nomotopic tachycardia and the role of the extrinsic cardiac nerves. Archives of Internal Medicine. 1921; 27(5):571-590.
- Wolff L. Bundle branch block with short PR interval in healthy young people prone to paroxysmal tachycardia. Am Heart J. 1930; 5:685-704.
- Kulig J, Koplan BA. Wolff-Parkinson-White syndrome and accessory pathways. Circulation. 2010;122(15):e480-e483.
- Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes NAM 3rd, Field ME, Goldberger ZD, Hammill SC, Indik JH, Lindsay BD, Olshansky B, Russo AM, Shen WK, Tracy CM, Al-Khatib SM. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-e115. doi: 10.1016/j.jacc.2015.08.856. Epub 2015 Sep 24. Erratum in: J Am Coll Cardiol. 2016 Dec 27;68(25):2922-2923. PMID: 26409259.
- Pediatric and Congenital Electrophysiology Society (PACES); Heart Rhythm Society (HRS); American College of Cardiology Foundation (ACCF); American Heart Association (AHA); American Academy of Pediatrics (AAP); Canadian Heart Rhythm Society (CHRS), Cohen MI, Triedman JK, Cannon BC, Davis AM, Drago F, Janousek J, Klein GJ, Law IH, Morady FJ, Paul T, Perry JC, Sanatani S, Tanel RE. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm. 2012 Jun;9(6):1006-24. doi: 10.1016/j.hrthm.2012.03.050. Epub 2012 May 10. PMID: 22579340.
- Kashou A WP, Kowlgi GNN. Asymptomatic Ventricular Preexcitation (Wolff-Parkinson-White Pattern): When to Be Concerned. American College of Cardiology. 2022.
- Cox JL, Gallagher JJ, Cain ME. Experience with 118 consecutive patients undergoing operation for the Wolff-Parkinson-White syndrome. J Thorac Cardiovasc Surg. 1985 Oct;90(4):490-501. PMID: 4046618.
- Cain ME, Luke RA, Lindsay BD. Diagnosis and localization of accessory pathways. Pacing Clin Electrophysiol. 1992 May;15(5):801-24. doi: 10.1111/j.1540-8159.1992.tb06847.x. PMID: 1382283.
- Ferguson T. Anatomic and electrophysiologic principles in the surgical treatment of cardiac arrhythmias. Cardiac arrhythmia surgery Philadelphia: Hanley and Belfus, Inc. 1990:19-51.
- Bardy GH, Packer DL, German LD, Gallagher JJ. Preexcited reciprocating tachycardia in patients with Wolff-Parkinson-White syndrome: incidence and mechanisms. Circulation. 1984 Sep;70(3):377-91. doi: 10.1161/01.cir.70.3.377. PMID: 6744541.
- Gallagher JJ, Pritchett EL, Sealy WC, Kasell J, Wallace AG. The preexcitation syndromes. Prog Cardiovasc Dis. 1978 Jan-Feb;20(4):285-327. doi: 10.1016/0033-0620(78)90015-4. PMID: 146210.
- Macedo PG, Patel SM, Bisco SE, Asirvatham SJ. Septal accessory pathway: anatomy, causes for difficulty, and an approach to ablation. Indian Pacing Electrophysiol J. 2010 Jul 20;10(7):292-309. PMID: 20680108; PMCID: PMC2907089.
- Jose Jalife WGS. Zipes and Jafile's Cardiac Electrophysiology-From Cell to Bedside. 2022(8th edition); 873.
- Hluchy J. Mahaim fibers: electrophysiologic characteristics and radiofrequency ablation. Z Kardiol. 2000;89 Suppl 3:136-43. doi: 10.1007/s003920050023. PMID: 10810796.
- Soares Correa F, Lokhandwala Y, Cruz Filho F, Sánchez-Quintana D, Mori S, Anderson RH, Wellens HJJ, Back Sternick E. Part II-Clinical presentation, electrophysiologic characteristics, and when and how to ablate atriofascicular pathways and long and short decrementally conducting accessory pathways. J Cardiovasc Electrophysiol. 2019 Dec;30(12):3079-3096. doi: 10.1111/jce.14203. Epub 2019 Oct 16. PMID: 31588593.
- Gupta A, Lokhandwala Y, Rai N, Malviya A. Adenosine-A drug with myriad utility in the diagnosis and treatment of arrhythmias. J Arrhythm. 2020 Dec 18;37(1):103-112. doi: 10.1002/joa3.12453. PMID: 33664892; PMCID: PMC7896475.
- Sethi KK, Dhall A, Chadha DS, Garg S, Malani SK, Mathew OP. WPW and preexcitation syndromes. J Assoc Physicians India. 2007 Apr;55 Suppl:10-5. PMID: 18368860.
- Boersma L, García-Moran E, Mont L, Brugada J. Accessory pathway localization by QRS polarity in children with Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 2002 Dec;13(12):1222-6. doi: 10.1046/j.1540-8167.2002.01222.x. PMID: 12521337.
- Narasimhan JFC. LeoSchamroth An Introduction to Electrocardiography. 2015(8th Edition); 194-196.
- Bhattad PB, Jain V. Revisiting Electrocardiographic Wolff-Parkinson-White Pattern. The Journal of Medical Research. 2020; 6(4):114-116.
- Guize L, Soria R, Chaouat JC, Chrétien JM, Houe D, Le Heuzey JY. Prévalence et évolution du syndrome de Wolff-Parkinson-White dans une population de 138 048 sujets [Prevalence and course of Wolf-Parkinson-White syndrome in a population of 138,048 subjects]. Ann Med Interne (Paris). 1985;136(6):474-8. French. PMID: 4083638.
- Vidaillet HJ Jr, Pressley JC, Henke E, Harrell FE Jr, German LD. Familial occurrence of accessory atrioventricular pathways (preexcitation syndrome). N Engl J Med. 1987 Jul 9;317(2):65-9. doi: 10.1056/NEJM198707093170201. PMID: 3587328.
- Munger TM, Packer DL, Hammill SC, Feldman BJ, Bailey KR, Ballard DJ, Holmes DR Jr, Gersh BJ. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989. Circulation. 1993 Mar;87(3):866-73. doi: 10.1161/01.cir.87.3.866. PMID: 8443907.
- Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979 Nov 15;301(20):1080-5. doi: 10.1056/NEJM197911153012003. PMID: 492252.
- Obeyesekere MN, Leong-Sit P, Massel D, Manlucu J, Modi S, Krahn AD, Skanes AC, Yee R, Gula LJ, Klein GJ. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation. 2012 May 15;125(19):2308-15. doi: 10.1161/CIRCULATIONAHA.111.055350. Epub 2012 Apr 24. PMID: 22532593.
- Etheridge SP, Escudero CA, Blaufox AD, Law IH, Dechert-Crooks BE, Stephenson EA, Dubin AM, Ceresnak SR, Motonaga KS, Skinner JR, Marcondes LD, Perry JC, Collins KK, Seslar SP, Cabrera M, Uzun O, Cannon BC, Aziz PF, Kubuš P, Tanel RE, Valdes SO, Sami S, Kertesz NJ, Maldonado J, Erickson C, Moore JP, Asakai H, Mill L, Abcede M, Spector ZZ, Menon S, Shwayder M, Bradley DJ, Cohen MI, Sanatani S. Life-Threatening Event Risk in Children With Wolff-Parkinson-White Syndrome: A Multicenter International Study. JACC Clin Electrophysiol. 2018 Apr;4(4):433-444. doi: 10.1016/j.jacep.2017.10.009. Epub 2017 Nov 15. PMID: 30067481.
- Chubb H, Ceresnak SR. A proposed approach to the asymptomatic pediatric patient with Wolff-Parkinson-White pattern. HeartRhythm Case Rep. 2020 Jan 15;6(1):2-7. doi: 10.1016/j.hrcr.2019.09.003. PMID: 31956492; PMCID: PMC6962761.
- Todd DM, Klein GJ, Krahn AD, Skanes AC, Yee R. Asymptomatic Wolff-Parkinson-White syndrome: is it time to revisit guidelines? J Am Coll Cardiol. 2003 Jan 15;41(2):245-8. doi: 10.1016/s0735-1097(02)02707-9. PMID: 12535817.
- Klein GJ, Gulamhusein SS. Intermittent preexcitation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1983 Aug;52(3):292-6. doi: 10.1016/0002-9149(83)90125-x. PMID: 6869275.
- Hindman MC, Last JH, Rosen KM. Wolff-Parkinson-White syndrome observed by portable monitoring. Ann Intern Med. 1973 Nov;79(5):654-63. doi: 10.7326/0003-4819-79-5-654. PMID: 4751741.
- Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J. 2001 Sep;142(3):530-6. doi: 10.1067/mhj.2001.117779. PMID: 11526369.
- Weng KP, Wolff GS, Young ML. Multiple accessory pathways in pediatric patients with Wolff-Parkinson-White syndrome. Am J Cardiol. 2003 May 15;91(10):1178-83. doi: 10.1016/s0002-9149(03)00263-7. PMID: 12745099.
- Santinelli V, Radinovic A, Manguso F, Vicedomini G, Gulletta S, Paglino G, Mazzone P, Ciconte G, Sacchi S, Sala S, Pappone C. The natural history of asymptomatic ventricular pre-excitation a long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009 Jan 20;53(3):275-80. doi: 10.1016/j.jacc.2008.09.037. PMID: 19147045.
- Escudero CA, Ceresnak SR, Collins KK, Pass RH, Aziz PF, Blaufox AD, Ortega MC, Cannon BC, Cohen MI, Dechert BE, Dubin AM, Motonaga KS, Epstein MR, Erickson CC, Fishberger SB, Gates GJ, Capone CA, Nappo L, Kertesz NJ, Kim JJ, Valdes SO, Kubuš P, Law IH, Maldonado J, Moore JP, Perry JC, Sanatani S, Seslar SP, Shetty I, Zimmerman FJ, Skinner JR, Marcondes L, Stephenson EA, Asakai H, Tanel RE, Uzun O, Etheridge SP, Janson CM. Loss of ventricular preexcitation during noninvasive testing does not exclude high-risk accessory pathways: A multicenter study of WPW in children. Heart Rhythm. 2020 Oct;17(10):1729-1737. doi: 10.1016/j.hrthm.2020.05.035. Epub 2020 Jun 1. PMID: 32497761.
- Bricker JT, Porter CJ, Garson A Jr, Gillette PC, McVey P, Traweek M, McNamara DG. Exercise testing in children with Wolff-Parkinson-White syndrome. Am J Cardiol. 1985 Apr 1;55(8):1001-4. doi: 10.1016/0002-9149(85)90734-9. PMID: 3984857.
- Gaita F, Giustetto C, Riccardi R, Mangiardi L, Brusca A. Stress and pharmacologic tests as methods to identify patients with Wolff-Parkinson-White syndrome at risk of sudden death. The American journal of cardiology. 1989;64(8):487-90.
- Wellens HJ, Bär FW, Gorgels AP, Vanagt EJ. Use of ajmaline in patients with the Wolff-Parkinson-White syndrome to disclose short refractory period of the accessory pathway. Am J Cardiol. 1980 Jan;45(1):130-3. doi: 10.1016/0002-9149(80)90230-1. PMID: 7350760.
- Wellens HJ, Braat S, Brugada P, Gorgels AP, Bär FW. Use of procainamide in patients with the Wolff-Parkinson-White syndrome to disclose a short refractory period of the accessory pathway. Am J Cardiol. 1982 Nov;50(5):1087-9. doi: 10.1016/0002-9149(82)90422-2. PMID: 7137035.
- Pappone C, Santinelli V, Rosanio S, Vicedomini G, Nardi S, Pappone A, Tortoriello V, Manguso F, Mazzone P, Gulletta S, Oreto G, Alfieri O. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. J Am Coll Cardiol. 2003 Jan 15;41(2):239-44. doi: 10.1016/s0735-1097(02)02706-7. PMID: 12535816.
- Leitch JW, Klein GJ, Yee R, Murdock C. Prognostic value of electrophysiology testing in asymptomatic patients with Wolff-Parkinson-White pattern. Circulation. 1990 Nov;82(5):1718-23. doi: 10.1161/01.cir.82.5.1718. Erratum in: Circulation 1991 Mar;83(3):1124. PMID: 2225373.
- Brembilla-Perrot B, Ghawi R. Electrophysiological characteristics of asymptomatic Wolff-Parkinson-White syndrome. Eur Heart J. 1993 Apr;14(4):511-5. doi: 10.1093/eurheartj/14.4.511. PMID: 8472715.
- Sharma AD, Yee R, Guiraudon G, Klein GJ. Sensitivity and specificity of invasive and noninvasive testing for risk of sudden death in Wolff-Parkinson-White syndrome. J Am Coll Cardiol. 1987 Aug;10(2):373-81. doi: 10.1016/s0735-1097(87)80021-9. PMID: 3598007.
- Moore JP, Kannankeril PJ, Fish FA. Isoproterenol administration during general anesthesia for the evaluation of children with ventricular preexcitation. Circ Arrhythm Electrophysiol. 2011 Feb;4(1):73-8. doi: 10.1161/CIRCEP.110.958660. Epub 2010 Dec 14. PMID: 21156771.
- Szabo TS, Klein GJ, Sharma AD, Yee R, Milstein S. Usefulness of isoproterenol during atrial fibrillation in evaluation of asymptomatic Wolff-Parkinson-White pattern. Am J Cardiol. 1989 Jan 15;63(3):187-92. doi: 10.1016/0002-9149(89)90283-x. PMID: 2909998.
- Willis P, Tabibiazar MR, Dave MR, Mazar M. Asymptomatic Patient with EKG showing Wolff-Parkinson-White Pattern. Proceedings of UCLA Healthcare. 2011; 15.
- Pappone C, Santinelli V, Manguso F, Augello G, Santinelli O, Vicedomini G, Gulletta S, Mazzone P, Tortoriello V, Pappone A, Dicandia C, Rosanio S. A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome. N Engl J Med. 2003 Nov 6;349(19):1803-11. doi: 10.1056/NEJMoa035345. PMID: 14602878.
- Bigelow AM, Smith G, Clark JM. Catheter Ablation Without Fluoroscopy: Current Techniques And Future Direction. J Atr Fibrillation. 2014 Apr 30;6(6):1066. doi: 10.4022/jafib.1066. PMID: 27957068; PMCID: PMC5135245.
- Alvarez M, Tercedor L, Almansa I, Ros N, Galdeano RS, Burillo F, Santiago P, Peñas R. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm. 2009 Dec;6(12):1714-20. doi: 10.1016/j.hrthm.2009.08.037. Epub 2009 Sep 3. PMID: 19959117.