More Information
Submitted: 03 March 2020 | Approved: 23 April 2020 | Published: 24 April 2020
How to cite this article: Allain F, Legallois D, Blanchart K, Champ-Rigot L, Pellissier A, et al. Effects of highest dose of sacubitril/valsartan association compared to lower doses on mortality and ventricular arrhythmias. J Cardiol Cardiovasc Med. 2020; 5: 089-094.
DOI: 10.29328/journal.jccm.1001092
Copyright License: © 2020 Allain F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Sacubitril/valsartan; Dose; Mortality; Ventricular arrhythmia; Implantable cardiac defibrillator
Abbreviations: SCD: Sudden Cardiac Death; Hfref: Reduced Ejection Fraction Heart Failure Patients; Acei: Angiotensin-Converting-Enzyme Inhibitor; ARB: Angiotensin Receptor Blocker; MRA: Mineraloid Receptor Antagonist; BBK: Beta-Blockers; Arni: Angiotensin-Neprilysin Inhibition (Sacubitril-Valsartan Association); ICD: Implantable Cardiac Defibrillator; CRT: Cardiac Resynchronization Therapy; VT/VF: Ventricular Tachycardia/Ventricular Fibrillation; ATP: Anti-Tachycardia Pacing; LVEF: Left Ventricular Ejection Fraction; VA: Ventricular Arrhythmias
Effects of highest dose of sacubitril/valsartan association compared to lower doses on mortality and ventricular arrhythmias
Florent Allain1#, Damien Legallois1,2#, Katrien Blanchart1, Laure Champ-Rigot1,2, Arnaud Pellissier1, Pierre Ollitrault1, Mathieu Chequel1, Rémi Sabatier1, Alain Lebon3, Sophie Gomes3, Olivier Citerne3, Farzin Beygui1,2 and Paul Milliez1.2*
1Department of Cardiology, Normandy University, UNICAEN, 14000 Caen, France
2Department of Cardiology, Normandy University, EA4650, Signalisation Electrophysiologie et Imagerie des Lésions D’ischémie-Reperfusion Myocardique (SEILIRM), FHU REMOD-VHF, 14000 Caen, France
3CHP Saint Martin, Service de rythmologie, Caen, France
#Both authors equally contributed
*Address for Correspondence: Paul MILLIEZ, MD, PhD, Department of Cardiology, Normandy University, Caen CHU, Avenue Cote de Nacre 14000, Caen, France, Tel: (+33)2-31-06-51-18; Fax: (+33)2-31-06-44-18; Email: milliez-p@chu-caen.fr
Background: Sudden cardiac death is a major healthcare issue in reduced ejection fraction heart failure (HFrEF) patients. Recently, the new association of sacubitril/valsartan showed a reduction of both ventricular arrhythmias (VA) and mortality even at low dose compared to enalapril in HF patients. The purpose of our study was to assess whether the highest dose of sacubitril/valsartan compared to lower doses may improve the rate of death and VA in a population of patients with HFrEF and with an implantable cardiac defibrillator (ICD).
Methods: 104 HF patients with reduced EF under sacubitril/valsartan with an ICD were divided in 2 groups: the first one with the lower doses of sacubitril/valsartan (24/26 mg or 49 mg/51 mg twice daily) and the second with the maximal dose (97mg/103mg twice daily). The primary outcome was a composite of death or appropriate ICD therapy for VA.
Results: After a median follow-up of 14 months, 39 patients were treated with lower doses and 65 patients with the highest dose. Patients from the lower doses group were older (70 [60-80] vs. 66 [60-70]; p = 0,03), more symptomatic at initiation (NYHA 3: 44% vs. 19%; p < 0,01) and more often in atrial fibrillation (31% vs. 12%; p = 0,04). The primary composite endpoint occurred in 14 patients (36%) in the low doses group versus 7 patients (11%) in high dose group (p < 0,01). This difference was particularly observed in the subgroup of patients with ischemic cardiomyopathy. In a multivariable analysis, the higher dose was independently associated with the primary outcome with an HR = 2,934 [IC 95% 1,147 – 7,504]; p = 0,03. Kaplan-Meier curve showed an early effect of the highest dose of sacubitril/valsartan association.
Conclusion: Patients with HFrEF under the highest dose of sacubitril/valsartan showed better clinical outcomes with a decrease of both mortality or appropriated ICD therapies related to ventricular arrhythmias.
Sudden cardiac death (SCD) is a major cause of mortality in reduced ejection fraction heart failure (HFrEF) patients [1]. It is now well-established that activation of both renin-angiotensin-aldosterone and adrenergic systems is harmful and favors the occurrence of heart failure (HF) and subsequent cardiovascular death. For many years, survival in patients with HFrEF improved thanks to pharmacological agents acting on neuro-hormonal activation (i.e. angiotensin-converting-enzyme inhibitor (ACEi), angiotensin receptor blocker (ARB), mineraloid receptor antagonist (MRA) and beta-blockers (BBK) [2].
Recently, a new pharmacological class combining angiotensin-neprilysin inhibition (sacubitril-valsartan association (ARNi)) has demonstrated a decrease of cardiovascular mortality compared to angiotensin inhibition alone in patients with HFrEF [3]. This mortality reduction was also observed in the sub-group of patients under “low dose” of ARNi compared to patients with a “low dose” of ACEi [4]. Interestingly, this mortality reduction was partially attributed to a reduction of SCD from unclear mechanisms. Several assumptions such as a reduction of cardiac fibrosis by the angiotensin inhibition and/or a modulation of myocardial electrophysiology related to the inhibition of natriuretic peptides degradation have been raised. A recent study in patients with implantable cardiac defibrillator (ICD) showed that the switch from angiotensin inhibition alone to ARNi led to a decrease of ventricular excitability, this reduction may partially underlie the beneficial effect of ARNi on SCD [5].
According to these recent data, we aimed to assess in patients with HFrEF and an ICD whether “highest dose” (97mg/103mg twice a day) compared to “lowest doses” (24 mg/26 mg and 49 mg/51 mg twice a day) of ARNi may decrease appropriate ICD therapies and improve clinical outcomes of patients with HFrEF.
Patient population and ARNi treatment initiation and titration
We retrospectively included 104 patients from January 2016 to September 2018. At the time of the inclusion, all patients had an ICD with or without a cardiac resynchronization therapy (CRT) with a clinical follow-up every 6-months after implantation. ICD were implanted in 2 centers in Normandy (CHU Caen and CHP Saint-Martin) for either primary or secondary prevention in patients with ischemic or non-ischemic cardiomyopathy. All patients had symptomatic HF characterized by a NYHA status ≥ 2 and a left ventricular ejection fraction (LVEF) ≤ 35% before implantation. All patients were considered on optimal medical therapies by the physicians before sacubitril/valsartan initiation with BBK, diuretics and MRA. The minimum follow-up after ARNi initiation and titration was 6 months, and the median follow-up with 14 months. Exclusion criteria were patients under ARNi less than 6 months before inclusion, patients with arrhythmogenic right ventricular cardiomyopathy, a left ventricular assist device or a heart transplant.
Initiation of ARNi started during hospitalization or during a clinical follow-up visit with progressive titration to the final tolerated dose as recommended. Then, patients were divided in 2 sub-groups according to their ARNi dose: “low doses” (24 mg/36 mg or 49 mg/51 mg twice a day) and “highest dose” (97 mg/103 mg twice a day). Patients were then followed every 6-months with ICD interrogation and clinical evaluation. Table 1 summarizes patient’s characteristics.
ICD therapy zones were programmed at the discretion of the electrophysiologist with the highest ventricular tachycardia/fibrillation (VT/VF) rate and optimal detection time to avoid appropriate antitachycardia pacing (ATP) and/or shocks [6]. All arrhythmic events and ICD therapies were collected during the follow-up visits by an electrophysiologist as recommended [6], and then re-analyzed and validated for the study by an independent and blinded electrophysiologist (PM). Appropriate therapy was defined as the occurrence of ATP or shock for VT/VF.
Clinical outcomes
The primary outcome was a composite outcome of mortality or appropriate ICD therapy. Then, each outcome was analyzed separately. Our secondary outcome focused on the difference between ischemic and non-ischemic patients.
Statistical analysis
Quantitative variables are expressed in median and interquartile range. Wilcoxon test was used to compare these variables. Qualitative data were analyzed using the exact Fisher test. Univariate analysis was performed using logistic regression. Multivariate analysis were carried out using a progressive selection algorithm (backward stepwise AIC) after integrating all parameters having a p < 0.15. Kaplan – Meier’s analysis was used for the representation of follow-up data on the main judgment criterion. The “Hazards Ratios” (HR) were calculated using the Cox model. A value of p < 0.05 was considered statistically significant. All statistical analyses were carried out using the software R, version 3.4.4.
Median follow-up was 14 months [12-15]. Among the 104 patients included in study, 39 patients (38%) were under « low doses » of ARNi (24/26 mg and 49 mg/51 mg twice a day) and 65 patients (62%) were under « highest dose » (97 mg/103 mg twice a day). Patients were predominantly male (89%) and in sinus rhythm (81%) with a median age of 67 [60-73] years old and with a mean LVEF of 30% [28-33]. Patients had predominantly ischemic cardiomyopathy (n = 68) and primary prevention represented the main ICD indication with a combined CRT device in 61% of the patients. At the time of ARNI optimization, other medical recommended therapies were not different between the two groups for BBK, ARM, oral diuretics and amiodarone. However, patients receiving the “low doses” were older, more symptomatic and more frequently had atrial fibrillation (Table 1).
| Table 1: Characteristics of the population. NYHA: New York Heart Association; LVEF: Left Ventricular Ejection Fraction; ICD: Implantable Cardioverter Defibrillator; MRA: Mineralo-Receptor Antagonist; CRT: Cardiac Resynchronization; VT: Ventricular Tachycardia | ||||
| Overall | Sacubitril/Valsartan “low doses” (24/26 mg and 49/51 mg) | Sacubitril/Valsartan “highest dose” (97/103 mg) | ||
| (n = 104) | n = 39) | (n = 65) | p - value | |
| Age, years | 67 [60-73] | 70 [60-80] | 66 [60-70] | 0.03 |
| Male, n(%) | 93 (89) | 34 (87) | 59 (90) | 0.75 |
| Follow-up (months) | 14 [12-15] | 13 [12-14] | 14 [11-16] | 0.04 |
| Cardiomyopathy | 0.4 | |||
| Ischemic, n(%) | 68 (65) | 28 (72) | 40 (61) | |
| Non-ischemic, n(%) | 36 (35) | 11 (28) | 25 (39) | |
| NYHA Class | <0.01 | |||
| II | 75 (72) | 22 (56) | 53 (81) | |
| III | 29 (28) | 17 (44) | 12 (19) | |
| Rhythm | ||||
| Sinus rhythm, n(%) | 84 (81) | 27 (69) | 57 (88) | 0.04 |
| Atrial fibrillation, n(%) | 20 (19) | 12 (31) | 8 (12) | |
| LVEF (%) | 30 [28-33] | 30 [27-33] | 30 [28-33] | 0.58 |
| ICD indication | 0.57 | |||
| Primary, n(%) | 90 (87) | 35 (90) | 55 (85) | |
| Secondary, n(%) | 14 (13) | 4 (10) | 10 (15) | |
| CRT, n(%) | 63 (61) | 26 (67) | 37 (57) | 0.41 |
After the follow-up period, while patients with the high dose had a significantly NYHA status improvement, LVEF increased similarly in both groups. There were no differences between the 2 groups for non-sustained VT and supraventricular tachycardia occurrence and inappropriate ICD therapies (Table 2).
| Table 2: Follow-up data. NYHA: New York Heart Association; LVEF: Left Ventricular Ejection Fraction; ICD: Implantable Cardioverter Defibrillator; MRA: Mineralo-Receptor Antagonist; CRT: Cardiac Resynchronization; VT: Ventricular Tachycardia | |||
| Sacubitril/Valsartan “low doses”(24/26 mg and 49/51 mg) | Sacubitril/Valsartan “highest dose” (97/103 mg) | ||
| (n = 39) | (n = 65) | ||
| NYHA Class | 0.04 | ||
| I | 7 (18) | 19 (29) | |
| II | 24 (61) | 43 (66) | |
| III | 8 (21) | 3 (5) | |
| LVEF (%) | 35 [30-38] | 36 [30-41] | 0.28 |
| ICD follow-up data | |||
| SVT, n(%) | 3 (8) | 10 (15) | 0.37 |
| NSVT, n(%) | 10 (26) | 15 (23) | 0.82 |
| Inappropriate therapies, n(%) | 1 (3) | 3 (5) | 1 |
| Resynchronization upgrade, n | 26 | 37 | 0.12 |
Primary outcome
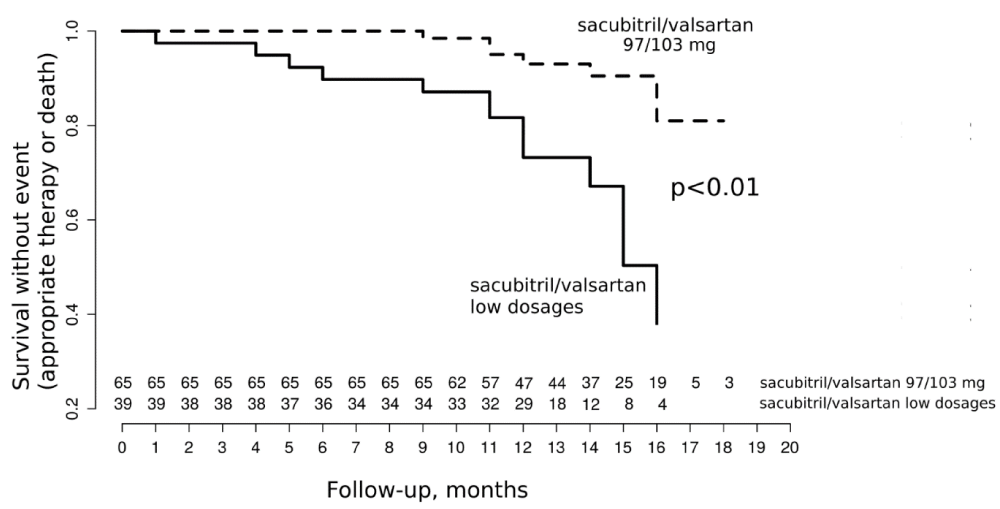
Primary composite outcome (mortality or appropriate therapy) occurred more often with low doses of ARNi than with highest dose with 14 (36%) vs. 7 events (11%) respectively, p < 0.01. This significant difference was driven by an important reduction of death in the high dose group (Table 3). Although not significant, there was less appropriate therapies in the high dose group (15% vs. 8%, p = 0.33). Interestingly, as illustrated in the Kaplan Meier model (Figure 1), this difference appeared early at 4 months after ARNi initiation. In univariate analysis, NYHA status, LVEF and highest dose of sacubitril-valsartan were associated with the primary outcome, while only LVEF and highest dose of sacubitril-valsartan remained associated with this primary outcome in multivariate analysis (Table 4).
| Table 3: Primary and secondary outcomes. | |||
| Sacubitril/Valsartan “low doses”(24/26 mg and 49/51 mg) | Sacubitril/Valsartan “highest dose” (97/103 mg) | p - value | |
| Primary composite outcome, n(%) | |||
| Overall | (n = 39) | (n = 65) | |
| Death or appropriate therapies | 14 (35.9) | 7 (10.8) | < 0.01 |
| Death | 10 (25.6) | 2 (3.1) | < 0.001 |
| Appropriate therapies | 6 (15.4) | 5 (7.7) | 0.33 |
| Patients with ischemic cardiomyopathy | ( n = 40) | (n = 28) | |
| Death or appropriate therapies | 13 (46.5) | 4 (10) | < 0.01 |
| Death | 10 (35.7) | 2 (5) | < 0.01 |
| Appropriate therapies | 5 (17.9) | 2 (5) | 0.12 |
| Table 4: Univariate and multivariate analysis. NYHA: New York Heart Association; LVEF: Left Ventricular Ejection Fraction; VT: Ventricular Tachycardia | ||||
| Univariate analysis Odds-ratio [CI 95%] | p - value | Multivariate analysis Odds-ratio (CI 95%) | p - value | |
| Age, per year | 1.067 [1.015-1.130] | 0.02 | ||
| Male gender | 1.155 [0.269-7.992] | 0.86 | ||
| Ischemic cardiomyopathy | 2.667 [0.892-9.902] | 0.11 | ||
| NYHA, per grade | 17.026 [5.656-59.975] | < 10-5 | 15.990 [5.114-59.148] | < 10-5 |
| Sacubitril/valsartan, per dosage | 0.366 [0.173-0.744] | < 0.01 | 0.389 [0.173-0.744] | 0.04 |
| LVEF (%) | 0.881 [0.793-0.972] | 0.02 | ||
| VT zone, per bpm | 0.983 [0.966-0.998] | 0.04 | ||
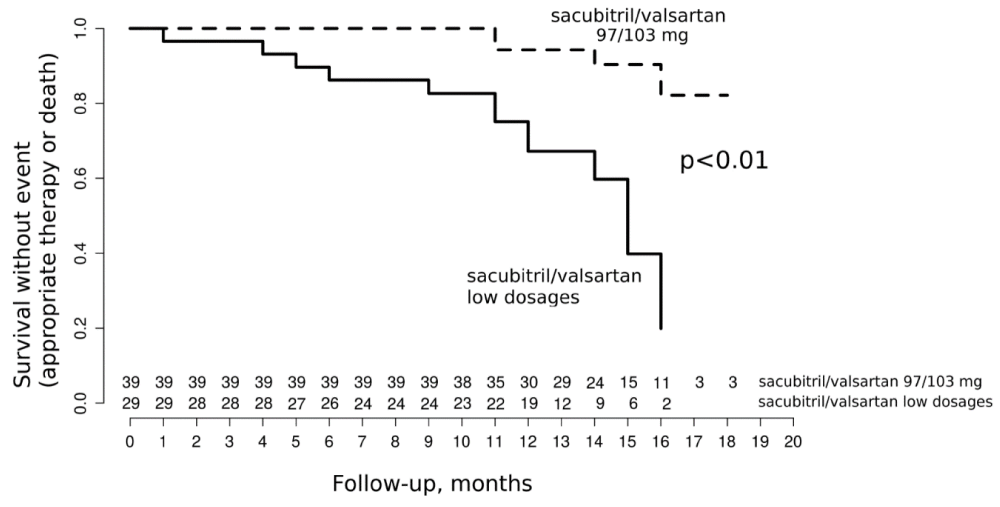
In addition, the sub-group analysis of patients with ischemic and non-ischemic cardiomyopathy showed that the primary outcome was only significantly different between the 2 ARNi dose groups in ischemic patients (Figure 2). This difference was higher than in the overall population with 13 (47%) vs. 4 events (10%) respectively for low doses and highest dose of ARNi (p < 0.01) and was also weighed by the reduction of mortality (Table 4).
Figure 1: Primary composite outcome of the study.
Figure 2: Composite outcome in patients with ischemic cardiomyopathy.
Our study showed that 1/highest dose of ARNi improves the composite endpoint of mortality or appropriate ICD therapy compared to lowest doses in a population of patients with HFrEF and an implanted ICD; 2/this improvement seemed to appear early after ARNi initiation and 3/was related to a significant reduction of death, particularly in ischemic patients.
The sacubitril/valsartan association is the first therapeutic combining a double inhibition of neprilysin and angiotensin pathways assessed in HFrEF patients in the PARADIGM-HF study [3]. The sacubitril/valsartan association initiated in patients with optimal medical treatment significantly reduced cardiovascular mortality; this reduction was observed mainly in patients in class I/II of NYHA even in patients with the low dose of ARNi [4]. In our study, we aimed to study whether patients on highest dose of ARNi have better clinical outcomes than those with the 2 lowest doses. Patients with the lower doses were older, more symptomatic and with higher rate of AF, but have similar LVEF, CRT implantation rates and medical treatment. However, these patients are likely more severe and optimization of sacubitril/valsartan association to the highest dose is somehow not possible and despite improvement in LVEF and clinical status with the low doses of ARNi, these patients in our study have poorer prognosis. This issue is of major concern because these older and more symptomatic patients with AF, again considered as more severe, may restrain ARNi titration because of theoretically less likelihood of ARNi optimization. Interestingly, our results showed that in the group with the highest dose, the beneficial effects seemed to be observed after 4 months, suggesting that failure to optimize sacubitril/valsartan association, either because clinically not suitable or not done, leads to worse outcomes. ARNi titration, even in severe patients, when clinically tolerated, should be undertaken as quickly as possible to have an impact on clinical outcomes.
In our study, we decided to combine mortality and occurrence of appropriate therapies for VT/VF as the primary clinical outcome because in the PARADIGM trial the mortality reduction was partially attributed to a reduction of SCD. Similarly to the recent publication from De Diego, et al. [5], we decided to include only patients with an ICD in order to analyze patient’s rhythm because ventricular arrhythmias (VA) are usually considered as the underlying mechanism of SCD in these patients. Our findings were similar to the results of the PARADIGM-HF trial [3], and from De Diego, et al. showing that the switch from angiotensin inhibition alone to ARNi led to a decrease of ventricular excitability [5]. However, we added further insights with better clinical outcomes, i.e. reduction of mortality or appropriate therapies, in patients with the highest dose of ARNi, and particularly in ischemic patients, the latter having a higher risk of VA than non-ischemic patients.
Improvement of clinical outcomes by ARNi, although not fully understood, is likely to be related to multiple complex mechanisms. First, valsartan is known to inhibit the cardiovascular and renal effects of angiotensin II by selectively blocking the AT1 receptors and aldosterone release. This inhibition of the renin-angiotensin-aldosterone system (RAAS) induces a vasodilation as well as decreased cardiac remodeling [7,8]. Sacubitril, a zinc-dependent metalloprotease enzyme type endopeptidase, inhibits the action of neprilysin by the LBQ 657. At the vascular level, the action of neprilysin is to hydrolyze endothelin and to inactivate peptides with vasodilator effects such as BNP as well as other proteins such as adrenomedullin and bradykinin. Inhibition of neprilysin by sacubitril allows natriuretic peptides to keep their vasodilatator effects by increasing their plasma levels. Altogether, it will induces vasodilation, increases renal blood flow, increases natriuresis, and inhibits RAAS and sympathetic systems [9]. At the cardiac level, neprilysin inhibition reduces myocardial inflammation, hypertrophy and fibrosis, leading to a reduction or reversion of myocardial remodeling [10]. Hence, these beneficial effects of valsartan/sacubitril combination on vascular as well as cardiac level may underlie the reduction in mortality observed in the PARADIGM-HF trial. However, the mechanisms of mortality reduction partially attributed to a reduction of SCD remain unclear. Clearly, reversion of the cardiac remodeling leads to a decrease in VT/VF substrate. Reduction of fibrosis and cardiomyocytes stretch may have direct electrophysiological actions. Myocardial fibrosis produces heterogeneity zones with areas of electrical slow conduction. Slow conduction area favors reentrant circuit representing the most frequent arrhythmias mechanism in patients with cardiomyopathy with and without HF. Increase of myocardial stretch leads to activation of stretch-induced channels modifying action potential characteristics and leading to increased risk of reentry and triggered activity [11,12]. In addition, other mechanisms have been evoked. Preliminary results in animal models showed that administration of sacubitril/valsartan following acute ischemia or reperfusion decreased the incidence of ventricular arrhythmias and in another study that the level of adrenaline peak was decreased by inhibition of endogenous degradation of enkephalins by racecadotril injection [13,14]. Altogether, these beneficial effects of the substrate, the trigger and the autonomic nervous system may lead to the reduction of SCD related to VA, especially in specific population of ischemic heart disease. In a clinical study, De Diego, et al. showed that sacubitril/valsartan reduces ventricular excitability recorded in patients with ICD and reduced ejection fraction [5]. We found in our study that reduction of our primary endpoint was particularly observed in our sub-group of ischemic patients, patients usually prone to have multiple triggers for ventricular arrhythmias.
Limitations
There are several limitations in our study. Although patients were followed prospectively, we retrospectively studied the clinical outcomes of the two sub-groups of patients. We included a limited number of patients, however, we observed a significant difference on mortality and a trends toward less ICD therapies in the high dose group. Because of the retrospective design, assessment of biomarkers correlated to mortality such as serum creatinine, BNP, or NT-pro-BNP levels have not been collected.
Combined therapy by sacubitril/valsartan at the highest dose shows beneficial clinical outcomes in patients having reduced ejection fraction heart failure compared to the 2 lowest doses. In addition, this effect seems to be observed early at 4 months, indicating that a rapid titration, when possible, is required. Patients with ischemic cardiomyopathy may particularly benefit from this association because of their specific myocardial substrate compared to non-ischemic patients. However, we need further prospective studies to confirm our preliminary results with larger and randomized dose-related study effects of ARNi on ventricular arrhythmias.
- Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, et al. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011; 57: 794-801. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21310315
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. 2016. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur J Heart Fail. 2016; 18: 891-975. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27207191
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, et al; PARADIGM-HF Investigators and Committees.Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. PubMed : https://www.ncbi.nlm.nih.gov/pubmed/25176015
- Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, et al. Efficacy of sacubitril/valsartan vs.. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. European Journal of Heart Failure, 2016; 18: 1228–1234. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27283779
- de Diego C, González-Torres L, Núñez JM, Centurión Inda R, Martin-Langerwerf DA, et al. Effects of angiotensin-neprilysin inhibition compared to angiotensin inhibition on ventricular arrhythmias in reduced ejection fraction patients under continuous remote monitoring of implantable defibrillator devices. Heart Rhythm. 2018; 15: 395–402. PubMed : https://www.ncbi.nlm.nih.gov/pubmed/29146274
- Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, et al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Europace. 2016; 18: 159–183. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26585598
- Webb RL, de Gasparo M. Role of the angiotensin II receptor blocker valsartan in heart failure. Exp Clin Cardiol. 2001; 6: 215-221. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2859003/
- Young JB. Mortality and Morbidity Reduction with Candesartan in Patients With Chronic Heart Failure and Left Ventricular Systolic Dysfunction: Results of the CHARM Low-Left Ventricular Ejection Fraction Trials. Circulation. 2004; 110: 2618–2626. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15492298
- Hubers SA, Brown NJ. Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition. Circulation. 2016; 133: 1115–1124. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26976916
- Iborra-Egea O, Gálvez-Montón C, Roura S, Perea-Gil I, Prat-Vidal C, et al. Mechanisms of action of sacubitril/valsartan on cardiac remodeling: a systems biology approach. Npj Systems Biology and Applications. 2017; 3.
- Sarrias A, Bayes-Genis A. Is Sacubitril/Valsartan (Also) an Antiarrhythmic Drug? Circulation. 2018; 138: 551–553. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30354612
- Franz MR, Cima R, Wang D, Profitt D, Kurz R. Electrophysiological Effects of Myocardial Stretch and Mechanical Determinants of Stretch-Activated Arrhythmias. Circulation. 1992; 86: 968-978. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1381296