More Information
Submitted: 13 March 2020 | Approved: 19 March 2020 | Published: 25 March 2020
How to cite this article: Özkan K, Mehdi K, KOBAT Mehmet A, Tarık K. Dapt Review. J Cardiol Cardiovasc Med. 2020; 5: 060-066.
DOI: 10.29328/journal.jccm.1001088
ORCiD: orcid.org/0000-0003-2896-6934
Copyright License: © 2020 Özkan K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Coronary arteries; Antiplatelets; Intervention
Dapt Review
KARACA Özkan*, KARASU Mehdi, KOBAT Mehmet A and KIVRAK Tarık
Faculty of Medicine, Cardiology, ABD, Fırat University, Turkey
*Address for Correspondence: Özkan KARACA, Faculty of Medicine, Cardiology, ABD, Fırat University, Turkey, Tel: 00905427783509; Email: md.ozkrc@gmail.com
Dual antiplatelet therapy (DAPT) combining aspirin and a P2Y12 receptor inhibitor has been consistently shown to reduce recurrent major adverse cardiovascular events (MACE) in patients with acute coronary syndrome (ACS) or undergoing percutaneous coronary intervention (PCI) for stable coronary artery disease (CAD) compared with aspirin monotherapy but at the expense of an increased risk of significant bleeding. Among patients with stable CAD undergoing PCI with drug-eluting stents (DES), shorter duration of DAPT (3–6 months) were shown non-inferior to 12 or 24 months duration concerning MACE but reduced the rates of major bleeding? Contrariwise, prolonged DAPT durations (18–48 months) reduced the incidence of myocardial infarction and stent thrombosis, but at the cost of an increased risk of majör bleeding and all-cause mortality. Until more evidence becomes available, the choice of optimal DAPT regimen and duration for patients with CAD requires a tailored approach based on the patient clinical presentation, baseline risk profile and management strategy. Patients with acute coronary syndromes (ACS) and a history of atrial fibrillation (AF) have indications for both dual antiplatelet therapy (DAPT) and oral anticoagulation (OAC). Triple therapy (TT), the combination of DAPT and OAC, is recommended in guidelines. This article provides a contemporary state-of-the-art review of the current evidence on DAPT for secondary prevention of patients with CAD and its future perspectives.
Platelet inhibition plays a central role in the treatment and prevention of short- and long-term atherothrombotic events in patients with coronary artery disease (CAD). Dual antiplatelet therapy (DAPT; a P2Y12 inhibitor [e.g., clopidogrel, prasugrel, ticagrelor] plus acetylsalicylic acid) is routinely given after percutaneous coronary intervention (PCI) with stenting to prevent stent thrombosis and majör adverse cardiovascular (CV) events (Levine et al., 2016). The recommended duration of DAPT for patients after drug-eluting stent (DES) implantation is ≥ 12 months for patients with the acute coronary syndrome (ACS), and six months for patients with stable coronary artery disease [1].
The recommendation for ≥ 12 months of DAPT after PCI with DES has received scrutiny by several randomised controlled trials, which proved nonsuperiority compared with three to six months of DAPT [2]. Furthermore, shorter durations, as opposed to longer durations of DAPT, were associated with lower rates of all-cause mortality as a result of lower rates of bleeding-related deaths [3].
The choice of optimal DAPT regimen and duration for patients with CAD requires a tailored approach based on the patient clinical presentation, baseline risk profile and management strategy. The American College of Cardiology/American Heart Association [4] and the European Society of Cardiology Guidelines [5], recommend tailoring the duration of DAPT based on patient characteristics.
Antiplatelet agents
Clopidogrel is associated with a better safety profile than ticlopidine, mainly in terms of allergy, skin or gastrointestinal disorders, and neutropenia. At the same time, it has a similar degree and consistency of P2Y12 inhibition and bleeding risk [6].
Prasugrel achieves a faster, greater, and more consistent degree of P2Y12 inhibition as compared to clopidogrel. Prasugrel requires two metabolic steps for formation of its active metabolite, which is chemically similar to the active metabolite of clopidogrel. Prasugrel was associated with a significant increase in the rate of non-CABG-related TIMI major bleeding (2.4% vs 1.8%; HR 1.32, 95% CI 1.03–1.68; p = 0.03). Life-threatening bleeding was significantly increased under prasugrel compared with clopidogrel (1.4% vs. 0.9%; HR 1.52, 95% CI 1.08–2.13; p = 0.01), as was fatal bleeding (0.4% vs. 0.1%, HR 4.19, 95% CI 1.58–11.11; p = 0.002). CABG-related bleeding was also higher in prasugrel-treated patients (13.4% vs. 3.2%; HR 4.72, 95% CI 1.90–11.82; p < 0.001). There was evidence of net harm with prasugrel in patients with a history of cerebrovascular events. Besides, there was no apparent net clinical benefit in patients >_75 years of age and patients with low body weight (< 60 kg) [7]. Hence, prasugrel is not indicated in patients with ACS in whom coronary anatomy is not known, and an indication for PCI is not clearly established, except for STEMI patients scheduled to undergo immediate coronary catheterization and PCI, if clinically indicated.
Ticagrelor belongs to a novel chemical class, cyclopentyl triazolopyrimidine, and is a direct oral, reversibly binding P2Y12 inhibitor with a plasma half-life of _12 h. In the PLATO trial, ticagrelor proved to be superior to clopidogrel in ACS patients, who were allowed to be pre-treated with clopidogrel at hospital admission, irrespective of the final revascularization strategy (i.e. planned or not planned invasive management) [8]. Patients with either moderate- to high-risk non-ST elevation ACS (NSTE-ACS) (planned for either conservative or invasive management) or STEMI planned for primary PCI were randomized to either clopidogrel 75mg daily, with a loading dose of 300mg, or ticagrelor 180 mg loading dose followed by 90 mg twice Daily [8]. Patients undergoing PCI were allowed to receive an additional blinded 300 mg loading dose of clopidogrel (total loading dose 600mg) or its placebo. They were also recommended to receive an additional 90 mg of ticagrelor (or its placebo) if > 24 h after the initial loading dose. The superiority of ticagrelor over clopidogrel concerning the primary study endpoint as well as cardiovascular death or overall mortality was consistent across management strategies, i.e. patients undergoing PCI, those medically managed, and patients who underwent CABG [8].
P2Y12 inhibitors in STEMI patients treated with lysis: Clopidogrel is the only P2Y12 inhibitor that has been properly investigated in patients with STEMI undergoing initial treatment with thrombolysis [9]. Clopidogrel 300 mg loading dose has been investigated only in patients <_75 years of age [9].
Stable CAD
In a large meta-analysis including 16 secondary prevention trials and 17 000 high-risk patients, low-dose aspirin (75–150 mg/day) was associated with a 20% relative risk reduction in MACE (cardiovascular (CV) death or non-fatal myocardial infarction (MI)) (rate ratio 0.80, 95% CI 0.73 to 0.88), a 31% relative risk reduction in MI (RR 0.69, 95% CI 0.60 to 0.80) and a 22% relative risk reduction in ischaemic stroke (RR 0.78, 95% CI 0.61 to 0.99) [10]. Aspirin marginally reduced CV mortality (RR 0.91, 95% CI 0.82 to 1.00, p = 0.06), resulting in a 10% relative risk reduction in all-cause mortality (RR 0.90, 95% CI 0.82 to 0.99, p = 0.02) [10]. The optimal risk: benefit ratio appears to be achieved with an aspirin dosage of 75–150 mg Daily [4,11].
The Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) trial compared antiplatelet therapy with clopidogrel (75 mg daily) versus aspirin (325 mg daily) in 19185 patients with atherosclerotic cardiovascular disease (ACVD) (recent ischaemic stroke, recent MI or symptomatic peripheral arterial disease (PAD)) [12]. Compared with aspirin, long-term administration of clopidogrel (median follow-up two years) was associated with significant risk reductions in the combined endpoint of CV death, MI or ischaemic stroke (5.32% per year vs. 5.83% per year, relative risk reduction 8.7%, 95% CI 0.3 to 16.5, p = 0.04) without significantly increased risk of severe intracranial (0.31% vs. 0.43%, p = 0.23) and gastrointestinal bleedings (0.49% vs. 0.71%, p = 0.05) [12]. Importantly, the superiority of clopidogrel over aspirin was mainly driven by a reduction of events in the PAD, but not MI, subgroup [12]. According to current guidelines, long-term low-dose aspirin is recommended in all patients with stable CAD (class I) [4,11]. Clopidogrel (75 mg daily) is indicated as an alternative in case of aspirin intolerance (class I) [4].
Routine DAPT is currently not recommended for patients with stable CAD without a history of ACS, PCI or CABG within 12 months (class III) [4,11]. Results from the CHARISMA trial suggest the potential benefits of DAPT with aspirin and clopidogrel beyond aspirin alone in a subgroup of patients with stable CAD and at high risk of CV events [13].
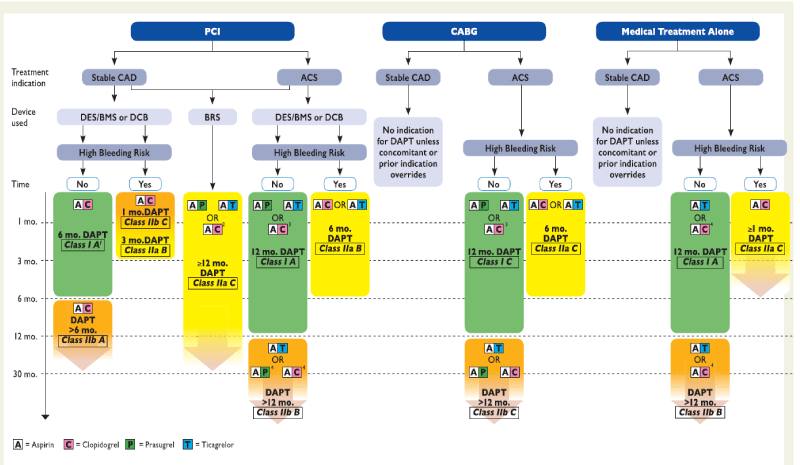
After the percutaneous coronary intervention (PCI), the combination of aspirin and P2Y12 receptor inhibitör therapy remains the mainstay of pharmacological treatment for patients undergoing PCI with bare-metal stents (BMS) or drug-eluting stents (DES). (Figure 1). Among patients undergoing PCI, DAPT with aspirin and a P2Y12 receptor antagonist (ticlopidine) during 4–6 weeks significantly reduced rates of MACE compared with combined aspirin and oral anticoagulation (OAC) therapy [14,15] or aspirin single antiplatelet therapy, and decreased majör bleeding rates compared with the combination of aspirin and OAC [14,15]. However, prolonged DAPT duration increases the risk of major bleeding compared with aspirin alone, which has been strongly related to an increased risk of short and long-term mortality [16].
Figure 1: Algorithm for DAPT in patients with coronary artery disease. ACS = acute coronary syndrome, BMS = bare-metal stent; BRS = bioresorbable vascular scaffold; CABG = Coronary artery bypass graft; DCB = drug-coated balloon; DES: drug-eluting stent; PCI = percutaneous coronary intervention; Stable CAD = stable coronary artery disease. High bleeding risk is considered as an increased risk of spontaneous bleeding during DAPT (e.g. PRECISE-DAPT score >_25). Colour-coding refers to the ESC Classes of Recommendations (green = Class I; yellow = Class IIa; orange = Class IIb). Treatments presented within the same line are sorted in alphabetic order, no preferential recommendation unless clearly stated otherwise.1: After PCI with DCB 6 months. DAPT should be considered (Class IIa B). 2: If a patient presents with Stable CAD or, in case of ACS, is not eligible for treatment with prasugrel or ticagrelor. 3: If the patient is not eligible for treatment with prasugrel or ticagrelor. 4: If the patient is not eligible for treatment with ticagrelor.
While there is consensus on 1-month DAPT duration after BMS implantation, [4,17] the optimal duration of DAPT after DES implantation remains a matter of debate.
In patients with all clinical presentations, firstly, long term DAPT led to a higher risk of non-cardiac death and significant bleeding than short term DAPT in patients, and the discrimination was more noticeable when restricting long term DAPT to ≥ 18 months. Secondly, myocardial infarction and stent thrombosis showed no apparent difference between the short term and standard term DAPT, and standard term DAPT increased the risk of any bleeding. Thirdly, the risk of non-cardiac death and bleeding increased synchronously with increasing durations of DAPT. Fourthly, all-cause mortality, cardiac death, stroke, and net adverse clinical events presented similar risks for the three durations.
Antiplatelet therapy with aspirin, preferably when initiated within 24 hours after CABG, has been shown to significantly improve early postoperative saphenous vein graft patency and reduce major adverse ischaemic events in patients undergoing surgical revascularization [18]. While aspirin administration remains a class I indication, the benefits of combined aspirin and clopidogrel therapy after CABG remain controversial [4].
Acute coronary syndrome
Antiplatelet therapy with aspirin remains the cornerstone of pharmacological therapy for patients with ACS, irrespective of the clinical setting (non-ST-elevation ACS (NSTE-ACS), or ST-elevation myocardial infarction (STEMI)) and the patient management strategy (conservative treatment, PCI or CABG) [4].
In the CURRENT-OASIS 7 trial including 25 086 patients with ACS, no significant difference was observed between high-dose (300–325 mg daily) and low-dose (75–100 mg daily) aspirin about the composite endpoint of CV death, MI or stroke at 30 days (4.2% vs. 4.4%, HR 0.97, 95% CI 0.86 to 1.09, p = 0.61), irrespective of the management strategy (conservative treatment or PCI) [19,20]. Low-dose aspirin was associated with significant lower rates of major gastrointestinal bleeding (0.2% vs. 0.4%, p = 0.04), whereas high-dose aspirin showed no reduction in rates of the primary endpoint (4.1% vs. 4.2%, HR 0.98, 95% CI 0.84 to 1.13, p = 0.76) or major bleeding (1.5% vs. 1.3%, HR 1.18, 95% CI 0.92 to 1.53, p = 0.20) in the subgroup of patients undergoing PCI [19,20]. Furthermore, a subanalysis of the PLATO trial has recently suggested a reduced efficacy of ticagrelor versus clopidogrel in ACS patients treated with high aspirin doses. In contrast, ticagrelor appeared to be more effective than clopidogrel in decreasing CV events in a patient on low-dose aspirin [21].
Current guidelines recommend DAPT combining low-dose aspirin (75–100 mg/day) and a P2Y12 receptor inhibitor (clopidogrel or ticagrelor) during 12 months for patients with ACS managed conservatively (class I) [4]. Although the use of ticagrelor over clopidogrel seems reasonable (class IIa), six the administration of prasugrel is not recommended (class III). Long term DAPT may be considered for selected patients who tolerated the DAPT regimen during the first 12 months without bleeding (class IIb) [4]. For patients with ACS (NSTE-ACS or STEMI) treated with DAPT and undergoing surgical revascularisation, current guidelines recommend to resume the P2Y12 receptor inhibitor therapy after CABG to complete the 12-month DAPT duration after ACS (class I) [4].
Current guidelines recommend DAPT with a P2Y12 receptor antagonist for one year after acute MI [4]. However, patients with prior MI remain at increased long-term risk for ischaemic events (CV death, MI or stroke) during the subsequent years. The potential benefit of extended duration of DAPT beyond one year for the long-term secondary prevention of CV events after MI remains a matter of debate.
Switching between oral P2Y12 inhibitors
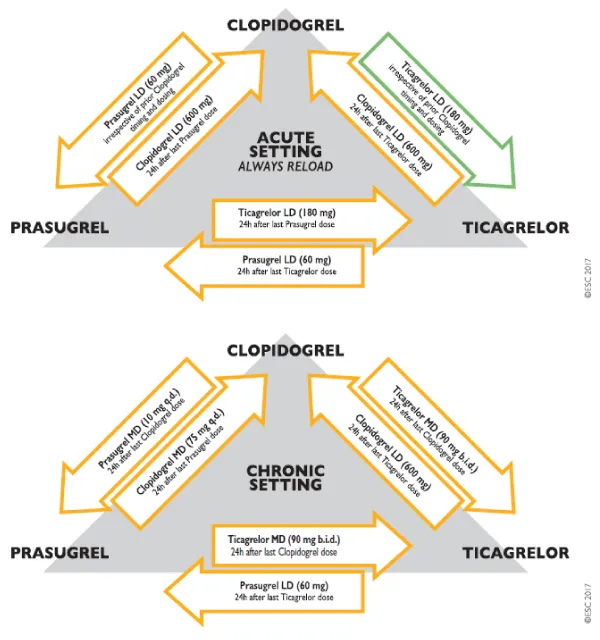
The transition from clopidogrel to ticagrelor is the only switch between P2Y12 inhibitors that has been investigated in a trial powered for the clinical endpoint, even if the study was not explicitly designed to assess the safety and efficacy of the transition from clopidogrel to ticagrelor. As the need to switch between P2Y12 inhibitors may arise for clinical reasons (i.e. side effects or drug intolerance), and registry data indicate that switching is not infrequent in practice, switching algorithms based on pharmacodynamic studies are provided (Figure 2).
Figure 2: Algorithm for switching between oral P2Y12 inhibitors in the acute and chronic setting. LD = loading dose; MD = maintenance dose. Colour-coding refers to the ESC Classes of Recommendations (green = Class I; orange = Class IIb). The green arrow from clopidogrel to ticagrelor highlights the only switching algorithm for which outcome data are available in patients with the acute coronary syndrome. No outcome data (orange arrows) are available for all other switching algorithms. The acute setting is considered as a switching occurring during hospitalization.
In patients with atrial fibrillation
Presentation with acute coronary syndromes (ACS) and concurrent AF is familiar with studies reporting between 6 and 21% of patients with ACS to have parallel AF. Patients presenting with both ACS and AF tend to be older, have more comorbidities and worse clinical outcomes [22]. Treatment with DAPT for one year is standard-of-care in those presenting with ACS and treatment with DAPT is superior to oral anticoagulants in those undergoing percutaneous coronary intervention (PCI) [23].
Current guidelines and consensus expert reports generally recommend individualizing therapy based on a patient’s ischaemic and bleeding risk and frequently recommend treatment with triple therapy (TT), a combination of DAPT and OAC therapy, in those with ACS and AF [24,25]. However the optimal treatment for AF patients with ACS, and the risks and benefits of TT compared with DAPT in this setting have not been established.
Based on the small number of studies in this systematic review, it is evident that bleeding rates are significantly higher in patients treated with TT compared to DAPT. This was demonstrated consistently in the adjusted results, including the two most extensive studies, Fosbol, et al. [26] and Lamberts, et al. [27], with the former particularly pertinent as it was the only study to only include patients with ACS. More considerable bleeding in TT groups was also supported in the majority of unadjusted results.
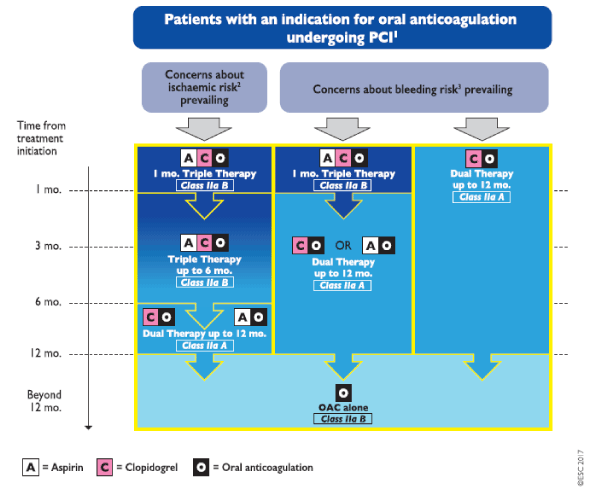
The ESC guideline for dual antiplatelet therapy (2017) notes a road map about the triple therapy (Figure 3).
Figure 3: Algorithm for dual antiplatelet therapy (DAPT) in patients with an indication for oral anticoagulation undergoing percutaneous coronary intervention (PCI). Colour-coding refers to the number of concomitant antithrombotic medication(s). Triple therapy denotes treatment with DAPT plus oral anticoagulant (OAC). Dual therapy denotes treatment with a single antiplatelet agent (aspirin or clopidogrel) plus. ABC = age, biomarkers, clinical history; ACS = acute coronary syndrome; mo. = month(s); PCI = percutaneous coronary intervention. 1: Periprocedural administration of aspirin and clopidogrel during PCI is recommended irrespective of the treatment strategy. 2: High ischaemic risk is considered as an acute clinical presentation or anatomical/procedural features which might increase the risk for myocardial infarction. 3: Bleeding risk can be estimated by HAS-BLED or ABC score.
Bleeding risk in this context is defined by HAS-BLED [28], and while this score has been well validated in AF, it has not been approved in AF and ACS. The current ACC/AHA STEMI [29] and NSTEMI [30] guidelines both note the increased risk of bleeding associated with TT and suggest that where this is warranted, an INR of 2.0 to 2.5 might be considered. The ACC/AHA guidelines do not reference a bleeding score. The studies included in the current review showed similar bleeding scores in both treatment arms, suggesting that bleeding risk was not strongly associated with treatment allocation. In three studies, there was a higher stroke risk in the TT arm, which may indicate stroke risk was a factor in treatment allocation in at least some cases.
TT was consistently associated with an increase in bleeding risk, but there was no consistent evidence of reduced stroke or reduced composite ischaemic endpoints related to TT. This review has highlighted the need for prospective randomized control trials to define optimal therapy and improve outcomes in the AF and ACS population.
Despite a large body of randomized evidence, the optimal regimen and duration of DAPT for secondary prevention of patients with CAD remains a matter of intense debate. Overall, evidence from available SRs supports a beneficial role of extended DAPT in reducing the risk of MI and stent thrombosis beyond 12 months after PCI with stenting. This is contrasted, however, by a potential increase in the risk of death and major bleeding, although previous reviews have reported conflicting findings. Future studies are needed to identify better patients who may derive benefit from either shortened or prolonged DAPT durations to improve outcomes while minimising bleeding risks.
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2017; 53: 34-78. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29045581
- Gwon HC1, Hahn JY, Park KW, Song YB, Chae IH, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012; 125: 505-513. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22179532
- Palmerini T, Bacchi Reggiani L1, Della Riva D1, Romanello M1, Feres F2, et al. Bleeding-related deaths in relation to the duration of dual-antiplatelet therapy after coronary stenting. J Am Coll Cardiol. 2017; 69: 2011-2022. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28427576
- Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016; 68: 1082-1115. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27036918
- Jeppsson A, Petricevic M, Kolh P, Valgimigli M. 2017 European Society of Cardiology (ESC) focused update on dual antiplatelet therapy in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Oxford University Press. 2017.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH; CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation. 2000; 102: 624-629. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10931801
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357: 2001-2015.
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361: 1045-1057. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19717846
- Sabatine MS, Cannon CP, Gibson CM, López-Sendón JL, Montalescot G, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005; 352: 1179-1189. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15758000
- Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials: Elsevier. 2009.
- Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F. et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013; 34: 2949-3003. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23996286
- Green D. CAPRIE trial. The Lancet. 349(9048). 354-355.
- Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007; 49: 1982-1988. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17498584
- Schömig A, Neumann FJ, Kastrati A, Schühlen H, Blasini R, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996; 334: 1084-1089. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8598866
- Urban P, Macaya C, Rupprecht HJ, Kiemeneij F, Emanuelsson H, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the multicenter aspirin and ticlopidine trial after intracoronary stenting (MATTIS). Circulation. 1998; 98: 2126-2132. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9815866
- Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, et al. Impact of bleeding on mortality after percutaneous coronary intervention: results from a patient-level pooled analysis of the REPLACE-2 (Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events), ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy), and HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trials. JACC: Cardiovascular Interventions. 2011 4: 654-664. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21700252
- Authors/Task Force members, Windecker S, Kolh P, Alfonso F, Collet JP, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014; 35: 2541-2619. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25173339
- Lorenz RL, Schacky CV, Weber M, Meister W, Kotzur J, et al. Improved aortocoronary bypass patency by low-dose aspirin (100 mg daily): effects on platelet aggregation and thromboxane formation. The Lancet. 1994; 323(8389). 1261-1264. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6144975
- Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. The Lancet. 2010; 376(9748): 1233-1243. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20817281
- CURRENT-OASIS 7 Investigators, Mehta SR, Bassand JP, Chrolavicius S, Diaz R, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010; 363: 930-942. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20818903
- Berger JS. Aspirin, clopidogrel, and ticagrelor in acute coronary syndromes. Am J Cardiol. 2013; 112: 737-745. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23751937
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014; 64: e1-e76. https://www.ncbi.nlm.nih.gov/pubmed/24685669
- Karjalainen PP1, Porela P, Ylitalo A, Vikman S, Nyman K, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J. 2007; 28: 726-732. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17267456
- Faxon DP, Eikelboom JW, Berger PB, Holmes DR, Bhatt DL, et al. Consensus document: antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting. Thrombosis and haemostasis. 2011; 106: 571-584. https://www.ncbi.nlm.nih.gov/pubmed/21785808
- Lip GY, Windecker S, Huber K, Kirchhof P, Marin F, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J. 2014; 35: 3155-3179. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25154388
- Fosbol EL, Wang TY, Li S, Piccini J, Lopes RD, et al. Warfarin use among older atrial fibrillation patients with non–ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J. 2013; 166: 864-870. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24176442
- Lamberts M, Gislason GH, Olesen JB, Kristensen SL, Schjerning Olsen AM, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol. 2013; 62: 981-989. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23747760
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010; 138: 1093-1100. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20299623
- O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 61: e78-e140. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23256914
- Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non–ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012; 60: 645-681. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22809746